-
PDF
- Split View
-
Views
-
Cite
Cite
Bertrand Audoin, Kryshani T. M. Fernando, Josephine K. Swanton, Alan J. Thompson, Gordon T. Plant, David H. Miller, Selective magnetization transfer ratio decrease in the visual cortex following optic neuritis, Brain, Volume 129, Issue 4, April 2006, Pages 1031–1039, https://doi.org/10.1093/brain/awl039
Close - Share Icon Share
Abstract
Patients with clinically isolated syndromes suggestive of multiple sclerosis have evidence for abnormality in normal appearing grey matter detected using the magnetization transfer ratio (MTR), a quantitative MRI measure. One potential mechanism for the decreased grey matter MTR (GM MTR) observed is trans-synaptic morphological abnormality secondary to demyelinating lesions that are in an anatomically linked pathway but remote location. We investigated this potential association by studying the location of abnormalities using voxel-based analysis of GM MTR maps in a group of 80 patients studied within 6 months of presenting with isolated optic neuritis and compared the findings with those seen in 50 age- and sex-matched healthy controls. Occipital cortex and whole brain analysis comparing all optic neuritis patients and controls revealed a selective decrease of MTR bilaterally in the visual cortex in patients [Brodmann area (BA) 17]. Whole brain analysis of patients fulfilling the McDonald criteria for multiple sclerosis (n = 20) showed a lower MTR compared to controls bilaterally in the visual cortex (BA 17/18), left hippocampus, bilateral superior temporal gyrus, bilateral lenticular nuclei and the right cerebellum. There was no significant difference in the percentage of grey matter between patients and controls in the regions of abnormal MTR detected in the visual cortex. The intrinsic MTR decrease seen in patients suggests that there are structural changes in the visual cortex following an attack of optic neuritis. Potential mechanisms for this include trans-synaptic neuronal degeneration and cortical synaptic morphological changes; such abnormalities may also contribute to MTR abnormalities observed in the normal appearing grey matter in multiple sclerosis.
Introduction
Multiple sclerosis is an inflammatory demyelinating disease of the human CNS. Although traditionally regarded as a white matter disease, abnormalities can also occur in grey matter as suggested by post-mortem pathological studies (Kidd et al., 1999; Peterson et al., 2001) and in vivo MRI studies (Miller et al., 2003). Sensitive magnetic resonance (MR) techniques, such as measures of cortical and grey matter atrophy (Filippi, 2001; Ge et al., 2001; De Stefano et al., 2003; Quarantelli et al., 2003; Sailer et al., 2003; Dalton et al., 2004; Tiberio et al., 2005), magnetic resonance spectroscopy (MRS) (Chard et al., 2002; Cifelli et al., 2002; Sharma et al., 2001; Wylezinska et al., 2003), diffusion tensor imaging (Ciccarelli et al., 2001; Bozzali et al., 2002; Rovaris et al., 2002) and magnetization transfer imaging (MTI) (Rovaris et al., 2000; Dehmeshki et al., 2003; Audoin et al., 2005; Davies et al., 2005) have demonstrated changes in the grey matter of multiple sclerosis patients. At least three mechanisms could potentially cause grey matter abnormalities in multiple sclerosis (Bjartmar and Trapp, 2001). First, although rarely visible on conventional MRI, demyelinating lesions frequently occur in the cortex and in other grey matter locations (Kidd et al., 1999; Peterson et al., 2001). Secondly, grey matter pathological changes might be the direct consequence of retrograde degeneration of axons passing through inflammatory/demyelinating white matter regions and extending to the corresponding neuronal cell body (Simon et al., 2000). Thirdly, there might be trans-synaptic neuronal morphological changes secondary to demyelinating lesions that are in an anatomically linked pathway but remote location (Evangelou et al., 2000). However, the actual mechanisms underlying MR-detected grey matter changes in multiple sclerosis—especially at the earliest stage of the disease when grey matter abnormalities already appear on quantitative MRI (Dalton et al., 2004; Davies et al., 2005; Fernando et al., 2005)—are largely unknown, since direct pathological correlation is rarely possible.
Studies of patients at the earliest stages of the disease—when there are usually relatively few white matter lesions present—might provide clarification into the potential mechanisms of grey matter abnormalities in multiple sclerosis. MTI appears particularly sensitive in detecting early abnormalities of normal appearing tissues in multiple sclerosis (Iannucci et al., 2000; Traboulsee et al., 2002; Audoin et al., 2004; Fernando et al., 2005). A recent study using magnetization transfer ratio (MTR) histogram analyses has demonstrated significant MTR reductions in the whole segmented grey matter soon after a clinically isolated syndrome (CIS) (Fernando et al., 2005). MTR reductions occurring specifically in the deep grey matter have also been detected at an early clinical stage of multiple sclerosis using statistical mapping analysis of grey matter MTR (GM MTR) maps (Audoin et al., 2004).
In approximately 85% of multiple sclerosis patients, the first clinical manifestation is a CIS, such as optic neuritis, brainstem or spinal cord syndromes. Optic neuritis results in an anatomically discrete lesion of the anterior visual pathway and, therefore, provides an opportunity to study whether anatomically remote grey matter abnormalities occur within the visual pathway as a consequence of the focal demyelinating optic nerve lesion. In particular, detection of abnormalities in the visual cortex would imply that trans-synaptic morphological changes occur. The present study therefore investigated patients with clinically isolated optic neuritis for the presence of grey matter structural abnormalities in the occipital cortex using a voxel-based analysis of GM MTR maps.
Methods
Subjects
Patients
We studied a group of 80 patients who presented within the previous 6 months with an episode of clinically isolated optic neuritis. Patients who underwent MTI were participating in a prospective, multiparameter, MR and clinical follow-up study of CIS patients first seen and investigated within 3 months of optic neuritis onset. Full details of the study design are provided elsewhere (Brex et al., 1999; Dalton et al., 2002). Appropriate investigations were undertaken to exclude alternative diagnoses. Disability was assessed using Kurtzke's Expanded Disability Status Scale (EDSS) (Kurtzke, 1983). The study had approval from the National Hospital for Neurology and Neurosurgery and Moorfields Eye Hospital ethics committees. Informed consent was obtained from all subjects before entry into the study. The MT study, performed in 80 patients, took place at the first scheduled follow-up visit 3 months after the baseline assessment. Visual acuity was measured using Snellen charts at both the baseline and 3 month follow-up visits.
Controls
Using identical methods, MTI was also obtained from 50 healthy adult controls matched for age and gender.
MRI protocol
All MR studies were performed on a 1.5 tesla GE Signa Echospeed scanner (General Electric Medical Systems, Milwaukee, WI, USA). The patients had baseline T2-weighted, dual-echo fast spin-echo (FSE) sequences and T1-weighted post-gadolinium (0.1 mmol/kg body weight) spin-echo sequences of the brain and spinal cord within 12 weeks of their initial presentation. The acquisition parameters were as follows: (i) brain—46 × 3 mm contiguous, axial oblique slices (parallel to the anterior/posterior commissural line; AC/PC line) covering the whole brain (matrix 256 × 256, FOV 24 × 18 cm, 1 NEX) with TR 3200 ms, TE 15/90 ms for the FSE T2-weighted sequence and TR 600 ms, TE 17 ms for the T1-weighted sequence; (ii) spinal cord—13 × 3 mm contiguous, sagittal slices (matrix 256 × 256, FOV 48 × 24 cm) with TR 2500 ms, TE 56/98 ms, two NEX for the FSE T2-weighted sequences and TR 500 ms, TE 19 ms, three NEX for the T1-weighted sequence. They then underwent a repeat MRI of the brain approximately 3 months after the baseline scan (mean 13 weeks, SD 2.2, range 8–20 weeks after baseline scan and a mean 19 weeks, SD 3.8, range 12–33 weeks after the initial CIS). Baseline and 3-month follow-up scans were analysed for the presence and number of T2 and gadolinium enhancing lesions by an experienced neuroradiologist blinded to the clinical data. After 3 months, the clinical and MRI findings were reviewed to determine whether patients fulfilled the McDonald diagnostic criteria for multiple sclerosis (McDonald et al., 2001).
T2 brain lesion load (T2LL) was measured from electronic images using a semiautomated contouring technique to outline lesions (Grimaud et al., 1996).
MTI sequence
MTI was obtained at the same time as the 3-month follow-up MRI scan, following CIS onset in 80 patients. A dual-echo proton density- and T2-weighted spin-echo sequence [28 × 5 mm contiguous, axial oblique slices (parallel to the AC/PC line)] covering the whole brain; TR 1720 ms, TE 30/80 ms, 0.75 NEX, matrix 256 × 128, FOV 24 × 24 cm, total acquisition time 20 min) was performed using an interleaved sequence described by Barker et al. (1996).
MT and non-MT images were acquired for both echo times; the interleaved nature of the sequence removes the need for coregistration of the images. The sequence was MT-weighted by the application of a pre-saturation pulse. The pre-saturation pulse was a Hamming apodized 3-lobe sinc pulse, with a duration of 64 ms, flip angle of 1430° and a peak amplitude of 14.6 μT, giving a nominal bandwidth of 62.5 Hz, applied 2 kHz from the water resonance.
The MTR is measured in percentage units (pu) and is calculated using the following formula, MTR = [(Mo−Ms)/Mo] × 100, where Mo and Ms are mean signal intensities without and with pre-saturation, respectively. MTR maps were calculated on a pixel-by-pixel basis using the short echo data because of its higher signal-to-noise ratio compared with the long echo data.
MTR maps were then co-registered onto the corresponding T2-weighted images of each subject. MTR maps were spatially normalized into the Montreal Neurological Institute (MNI) space using the T1 anatomical template provided in the SPM2 software (Wellcome Functional Imaging Laboratory, Institute of Neurology, London). A spatial normalization algorithm preserved voxel intensities (concentrations) even in regions where values had been stretched by warping (Ashburner and Friston, 2000; Good et al., 2001). The spatial transformation was also applied onto the T2 lesion mask. Then the normalized T2 lesion mask was applied onto the normalized MTR maps to obtain normalized MTR maps of T2 lesions. The difference between normalized MTR images and normalized MTR maps of T2 lesions gave the normal appearing brain (NAB) MTR map. After segmentation of the normalized NAB MTR maps using voxel intensities and a priori knowledge procedure (SPM 2), three maps—representing fractions of grey matter, normal appearing white matter (NAWM) and CSF—were obtained. Voxels with a percentage of grey matter greater than 75% were used to mask the normalized NAB MTR map, in order to obtain the normalized GM MTR map (nGM MTR).
Statistical mapping analysis
Before statistical comparison, nGM MTR maps were smoothed using a 6 mm Gaussian filter to minimize remaining spatial difference between subjects and to better satisfy conditions of the random field theory (Friston et al., 1995).
A hypothesis-driven region of interest approach was used to investigate for MTR abnormalities within the occipital cortex. A two-sample t-test was used to compare the nGM MTR maps of all CIS patients versus controls on a voxel-by-voxel basis after small volume correction (SVC) for the occipital cortex. ANOVA (analysis of variance) was used to compare the nGM MTR maps of the following subgroups: (i) patients fulfilling the McDonald criteria for multiple sclerosis; (ii) patients not fulfilling the criteria for multiple sclerosis; (iii) controls. Similarly, ANOVA was also used to compare the nGM MTR maps of patient subgroups with and without visible T2 lesions and controls. The same ANOVA comparison was also made in patient subgroups with and without optic radiation lesions and controls, and in patient subgroups with and without gadolinium enhancing lesions. False discovery rate estimations were used to correct for multiple comparisons, and a probability of 0.05 was considered to be significant. Clusters were located after transformation of MNI coordinates into Talairach space (Talairach and Tournoux, 1988) using a non-linear transformation (www.mrc-cbu.cam.ac.uk/imaging/mnispace.htlm).
In addition, a whole brain analysis was performed to investigate for the presence of other regions of abnormal GM MTR in all patients versus controls using a two-sample t-test, with amplitude threshold P < 0.001. The patient subgroups as above and controls were also compared using ANOVA with amplitude threshold P < 0.001.
In the nGM MTR maps, the number of voxels that are classified as containing more than 75% grey matter within any given region of interest may differ between subjects according to local variations in the amount of grey matter. After the smoothing stage required by the analysis procedure, the given region of interest will therefore have an actual proportion of grey matter that is influenced by the number of voxels included that contain <75% grey matter (these are counted as empty voxels when MTR is measured from the whole region of interest). Consequently, it is necessary to evaluate the mean percentage of grey matter for each subject in the region of interest that appears different on MTR in order to clarify that the GM MTR differences observed between patients and controls are not due to regional grey matter atrophy rather than intrinsic grey matter abnormality. In order to evaluate the mean percentage of grey matter in the regions of abnormal MTR, T2 scans of all subjects were segmented using the mutual information procedure (SPM 2, Wellcome Functional Imaging Laboratory, Institute of Neurology, London). Grey matter maps were then smoothed using the same level Gaussian filter (6 mm) as for the MTR maps. This made it possible to calculate the percentage assigned as grey matter in the regions that appeared different on MTR. A statistical t-test comparison was then used to compare local percentages of grey matter between patients and controls.
Results
Clinical demographics
Clinical characteristics of the patients are summarized in Table 1. Fifty-two of the CIS patients were female and 28 were male. Their median age was 32 years (range 19–49 years) and median score on the EDSS was 1 (range 0–4). At the baseline assessment, no patient exhibited clinical signs suggestive of functional system involvement outside the optic nerve: consequently, individual EDSS scores were only related to visual function impairment. The median visual acuity of the symptomatic eye was 6/18 (range 0–6/4) at the baseline assessment and 6/6 (range 6/60–6/4) at 3-months follow-up when MTR imaging was performed. The median age for the controls was 33 years (range 23–53 years), with 22 males and 28 females. Twenty patients fulfilled the McDonald criteria for multiple sclerosis at the time MTR was performed. Sixteen patients had no visible T2 lesions and eighteen patients had gadolinium enhancing lesions.
Clinical characteristics of patients
. | All CIS patients (n = 80) . | Patients fulfilling McDonald's criteria (n = 20) . | Patients not fulfilling McDonald's criteria (n = 60) . |
|---|---|---|---|
| Age, mean (range) | 32 (19–49) | 35 (22–49) | 31 (19–48) |
| EDSS, median (range) | 1 (0–4) | 1 (0–3) | 1 (0–4) |
| Time since the optic neuritis onset in weeks, mean (SD) | 19 (3.9) | 18.4 (2.8) | 19.2 (4.2) |
| T2LL, mean (SD), cm3 | 3.9 (6.2) | 9.7 (7.6) | 2 (0.8) |
. | All CIS patients (n = 80) . | Patients fulfilling McDonald's criteria (n = 20) . | Patients not fulfilling McDonald's criteria (n = 60) . |
|---|---|---|---|
| Age, mean (range) | 32 (19–49) | 35 (22–49) | 31 (19–48) |
| EDSS, median (range) | 1 (0–4) | 1 (0–3) | 1 (0–4) |
| Time since the optic neuritis onset in weeks, mean (SD) | 19 (3.9) | 18.4 (2.8) | 19.2 (4.2) |
| T2LL, mean (SD), cm3 | 3.9 (6.2) | 9.7 (7.6) | 2 (0.8) |
Clinical characteristics of patients
. | All CIS patients (n = 80) . | Patients fulfilling McDonald's criteria (n = 20) . | Patients not fulfilling McDonald's criteria (n = 60) . |
|---|---|---|---|
| Age, mean (range) | 32 (19–49) | 35 (22–49) | 31 (19–48) |
| EDSS, median (range) | 1 (0–4) | 1 (0–3) | 1 (0–4) |
| Time since the optic neuritis onset in weeks, mean (SD) | 19 (3.9) | 18.4 (2.8) | 19.2 (4.2) |
| T2LL, mean (SD), cm3 | 3.9 (6.2) | 9.7 (7.6) | 2 (0.8) |
. | All CIS patients (n = 80) . | Patients fulfilling McDonald's criteria (n = 20) . | Patients not fulfilling McDonald's criteria (n = 60) . |
|---|---|---|---|
| Age, mean (range) | 32 (19–49) | 35 (22–49) | 31 (19–48) |
| EDSS, median (range) | 1 (0–4) | 1 (0–3) | 1 (0–4) |
| Time since the optic neuritis onset in weeks, mean (SD) | 19 (3.9) | 18.4 (2.8) | 19.2 (4.2) |
| T2LL, mean (SD), cm3 | 3.9 (6.2) | 9.7 (7.6) | 2 (0.8) |
Statistical mapping analysis of GM MTR maps
Occipital cortex GM MTR analysis
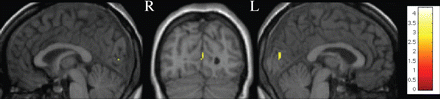
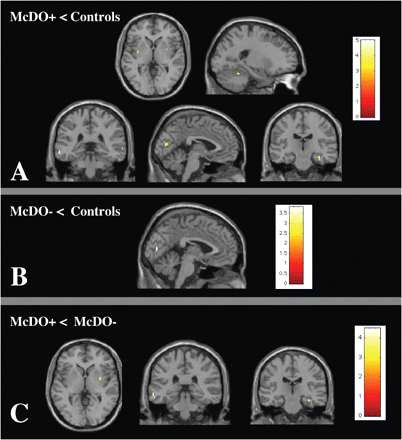
The t-test comparison between all patients (n = 80) and controls (n = 50) demonstrated a significant decrease of GM MTR in patients bilaterally in the visual cortex [Brodmann area (BA) 17] (Fig. 1) (two-sample t-test, corrected for multiple comparisons at the level P < 0.05, SVC analysis for the occipital cortex). Patients fulfilling the McDonald criteria (n = 20) had lower MTR values compared to controls (n = 50) bilaterally in the visual cortex (BA 17/18) (ANOVA analysis, corrected for multiple comparisons at the level P < 0.05, SVC analysis for the occipital cortex) (Fig. 2). Comparison between patients not fulfilling McDonald criteria (n = 60) versus controls (n = 50) demonstrated GM MTR decrease located bilaterally in the visual cortex (BA 17) (ANOVA analysis, corrected for multiple comparisons at the level P < 0.05, SVC analysis for the occipital cortex) (Fig. 2). There was no occipital GM MTR difference between patients who did or did not fulfil the McDonald criteria.
Voxel-by-voxel whole brain GM MTR maps comparison between CIS patients with optic neuritis (n = 80) and controls (n = 50) (two-sample t-test, amplitude threshold P < 0.001). Results are displayed on a normalized T1 scan. MTR decrease in patients is observed bilaterally in the visual cortex (BA 17).
Voxel-by-voxel whole brain GM MTR maps comparison between CIS patients fulfilling McDonald's criteria (n = 20) and controls (n = 50) (A), CIS patients not fulfilling McDonald's criteria (n = 60) and controls (n = 50) (B), CIS patients fulfilling McDonald's criteria (n = 20) and CIS patients not fulfilling McDonald's criteria (n = 60) (C) (ANOVA analysis, amplitude threshold P < 0.001). A: MTR decreases are located in the bilateral visual cortex (BA 17/18), the bilateral lenticular nuclei, the bilateral superior temporal cortex (BA 21), the right cerebellum and the left hippocampus. B: MTR decreases are located in the bilateral visual cortex (BA 17). C: MTR decreases are located in the left lenticular nucleus, the right superior temporal cortex (BA 21) and the left hippocampus.
Patients with T2 lesions (n = 64) exhibited a significant MTR decrease bilaterally in the visual cortex (BA 17) compared to controls (n = 50) (ANOVA analysis, corrected for multiple comparisons at the level P < 0.05, SVC analysis for the occipital cortex). Patients with no visible T2 lesions (n = 16) showed no significant GM MTR decrease in any part of the occipital cortex. Comparison between patients with and without T2 lesions demonstrated no significant difference.
Patients with optic radiation lesions (n = 43) and without optic radiation lesions (n = 37) both showed a significant MTR decrease in the visual cortex compared to controls (ANOVA analysis, corrected for multiple comparisons at the level P < 0.05, SVC analysis for the occipital cortex). There was no difference of MTR in this region between patients with and without optic radiation lesions. In addition, MTR was measured in two symmetrical regions of interest (ROIs) placed in the optic radiations on the normalized scans in order to study the potential correlation between MTR value in the optic radiations and the GM MTR in the occipital cortex in patients. The ROIs consisted in two circles of 32 voxels with respective centres located at −30 −70 +12 and +30 −70 +12 (Talairach space). This analysis demonstrated significant MTR decrease in the optic radiations in patients compared to controls (mean 36.7 ± 1.24 versus 37.1 ± 0.98; t-test, P = 0.026) but no correlation in patients between MTR of the optic radiations and the GM MTR in the visual cortex (Spearman rank correlation).
Finally, comparisons between patients with (n = 18) and without (n = 62) gadolinium enhancing lesions demonstrated no significant difference.
Whole brain GM MTR analysis
This analysis demonstrated selective GM MTR decrease located bilaterally in the visual cortex (BA 17) in patients with optic neuritis (n = 80) (two-sample t-test, amplitude threshold P < 0.001) (Fig. 1).
Patients fulfilling the McDonald criteria (n = 20) had lower MTR values compared to controls (n = 50) bilaterally in the visual cortex (BA 17/18), the left hippocampus, the bilateral superior temporal cortex (BA 21), the right cerebellum and the bilateral lenticular nuclei (Fig. 2) (ANOVA analysis, amplitude threshold P < 0.001). Comparison of patients not fulfilling the McDonald criteria (n = 60) versus controls (n = 50) demonstrated a selective GM MTR decrease located bilaterally in the visual cortex (BA 17) (Fig. 2) (ANOVA analysis, amplitude threshold P < 0.001). Patients fulfilling the McDonald criteria (n = 20) had a lower MTR in the left hippocampus and the left lenticular nucleus and the right superior temporal cortex (BA 21) compared to patients not fulfilling the McDonald criteria (n = 60) (ANOVA analysis, amplitude threshold P < 0.001) (Fig. 2).
Patients with T2 lesions (n = 64) exhibited a significant MTR decrease bilaterally in the visual cortex (BA 17/18) compared to controls (n = 50) (ANOVA analysis, amplitude threshold P < 0.001). Patients with no visible T2 lesions (n = 16) showed no significant GM MTR decreases in any part of the brain compared to controls. Comparison between patients with and without T2 lesions also demonstrated no significant differences.
Finally, comparisons between patients with (n = 18) and without (n = 62) enhancing lesions demonstrated no differences.
Percentage of GM in the regions of abnormal MTR (Table 2)
When all patients (n = 80) were compared to controls (n = 50), there was no significant difference seen in the percentage of GM in the region where abnormal MTR was present (P = 0.4). In the subgroup of patients fulfilling the McDonald criteria, the percentage of GM was reduced in the right lenticular nucleus compared to controls (P = 0.04).
GM fraction in the regions that appear different in MTR. T2 scans were segmented (SPM2) and percentage of GM was determined on the GM map after performing a smooth using the same level Gaussian filter (6 mm) as MTR maps
| GM MTR groups comparisons . | Regions of MTR decrease . | % GM . | . | . | ||
|---|---|---|---|---|---|---|
. | . | Controls . | Patients . | t-test . | ||
| CIS < controls | Bilateral visual cortex | 60 | 61 | P = 0.4 | ||
| McDO+ < controls | Left hippocampus | 77.4 | 76.2 | P = 0.58 | ||
| Left superior temporal gyrus | 74.4 | 73.8 | P = 0.76 | |||
| Right superior temporal gyrus | 71.2 | 67.7 | P = 0.06 | |||
| Right lenticular nucleus | 74.7 | 70 | P = 0.04 | |||
| Left lenticular nucleus | 55.8 | 53 | P = 0.35 | |||
| Bilateral visual cortex | 56.6 | 59.7 | P = 0.36 | |||
| McDO− < controls | Bilateral visual cortex | 60 | 58.2 | P = 0.5 | ||
| GM MTR groups comparisons . | Regions of MTR decrease . | % GM . | . | . | ||
|---|---|---|---|---|---|---|
. | . | Controls . | Patients . | t-test . | ||
| CIS < controls | Bilateral visual cortex | 60 | 61 | P = 0.4 | ||
| McDO+ < controls | Left hippocampus | 77.4 | 76.2 | P = 0.58 | ||
| Left superior temporal gyrus | 74.4 | 73.8 | P = 0.76 | |||
| Right superior temporal gyrus | 71.2 | 67.7 | P = 0.06 | |||
| Right lenticular nucleus | 74.7 | 70 | P = 0.04 | |||
| Left lenticular nucleus | 55.8 | 53 | P = 0.35 | |||
| Bilateral visual cortex | 56.6 | 59.7 | P = 0.36 | |||
| McDO− < controls | Bilateral visual cortex | 60 | 58.2 | P = 0.5 | ||
GM fraction in the regions that appear different in MTR. T2 scans were segmented (SPM2) and percentage of GM was determined on the GM map after performing a smooth using the same level Gaussian filter (6 mm) as MTR maps
| GM MTR groups comparisons . | Regions of MTR decrease . | % GM . | . | . | ||
|---|---|---|---|---|---|---|
. | . | Controls . | Patients . | t-test . | ||
| CIS < controls | Bilateral visual cortex | 60 | 61 | P = 0.4 | ||
| McDO+ < controls | Left hippocampus | 77.4 | 76.2 | P = 0.58 | ||
| Left superior temporal gyrus | 74.4 | 73.8 | P = 0.76 | |||
| Right superior temporal gyrus | 71.2 | 67.7 | P = 0.06 | |||
| Right lenticular nucleus | 74.7 | 70 | P = 0.04 | |||
| Left lenticular nucleus | 55.8 | 53 | P = 0.35 | |||
| Bilateral visual cortex | 56.6 | 59.7 | P = 0.36 | |||
| McDO− < controls | Bilateral visual cortex | 60 | 58.2 | P = 0.5 | ||
| GM MTR groups comparisons . | Regions of MTR decrease . | % GM . | . | . | ||
|---|---|---|---|---|---|---|
. | . | Controls . | Patients . | t-test . | ||
| CIS < controls | Bilateral visual cortex | 60 | 61 | P = 0.4 | ||
| McDO+ < controls | Left hippocampus | 77.4 | 76.2 | P = 0.58 | ||
| Left superior temporal gyrus | 74.4 | 73.8 | P = 0.76 | |||
| Right superior temporal gyrus | 71.2 | 67.7 | P = 0.06 | |||
| Right lenticular nucleus | 74.7 | 70 | P = 0.04 | |||
| Left lenticular nucleus | 55.8 | 53 | P = 0.35 | |||
| Bilateral visual cortex | 56.6 | 59.7 | P = 0.36 | |||
| McDO− < controls | Bilateral visual cortex | 60 | 58.2 | P = 0.5 | ||
Correlations between clinical data and GM MTR decrease in the visual cortex in the whole group of optic neuritis patients
The mean regional GM MTR was determined for each subject in the region surviving between-group random effect comparisons located in the visual cortex. Regional individual data were used to study correlations of visual cortex MTR in patients with T2LL, EDSS, time from onset of optic neuritis, and visual acuity at baseline and 3 month follow-up (Spearman rank correlation). The GM MTR in the visual cortex was correlated with the visual acuity at baseline (Spearman rank correlation, rs = 0.31, P = 0.011), after 3 months (Spearman rank correlation, rs = 0.30, P = 0.011) and with the EDSS (Spearman rank correlation, rs = −0.37, P = 0.001). No correlation was found between MTR in the visual cortex and T2LL or time from onset of optic neuritis.
Discussion
Using the statistical mapping approach applied to GM MTR data, a selective GM MTR decrease was identified in the visual cortex in patients with optic neuritis. The method used in this study has been recently described in studies of smaller cohorts with early but established multiple sclerosis and with heterogeneous clinical features (Audoin et al., 2004; Ranjeva et al., 2005). The advantage of this methodological approach is to enable—without generating a prior hypothesis about the location of abnormality—an unbiased comparison of quantitative parameters derived from various imaging modalities in all parts of the brain (Rugg-Gunn et al., 2003; Sailer et al., 2003; Ashburner and Friston, 2000).
It should be noted that the voxel-based analysis of GM MTR provides distinctly different information from what we have reported using global GM MTR histogram analyses in a large CIS cohort that included many of the same patients with optic neuritis (Fernando et al., 2005). Voxel-based analysis identifies focal grey matter regions in which there is a consistent MTR difference between two groups, i.e. it is likely that many patients in the optic neuritis group have decreased MTR in the visual cortex; however, it is not likely to identify GM MTR decreases that are inconsistently and variably located elsewhere in GM, e.g. if demyelinating grey matter lesions are more randomly scattered amongst different subjects elsewhere in the cortex. In contrast, global GM MTR histogram analysis will be influenced by GM abnormalities wherever they occur. The present voxel-based analysis does not exclude the probability that grey matter abnormalities already exist in optic neuritis patients outside the visual cortex: it does establish that there is a consistent abnormality within the visual cortex of such patients that is, therefore, likely to be linked to the occurrence of optic neuritis per se. Moreover the multiple transformations used during the pre-analysis processing may reduce the sensitivity of the comparisons, and consequently voxel-based analysis of MTR maps may demonstrate only regions where more substantial changes are present in patients.
Before addressing the pathological implications of the results, it is important to consider the potential for effects from atrophy and/or partial volume averaging to have influenced the main finding. Thus, focal GM atrophy in patients (compared to controls) could increase the proportion of CSF in voxels studied and thereby produce a decrease in MTR; in addition, inclusion of different amounts of white matter within the voxels could influence the findings, since white matter MTR differs between patients with CISs and controls (Fernando et al., 2005). In an effort to avoid these confounds, we selected for analysis only those voxels with at least 75% grey matter, accepting that this would discard some voxels that contained predominantly grey matter (i.e. >50% but <75%) and hence reduce sensitivity. Nevertheless, after smoothing the GM MTR maps, the MTR of the selected abnormal region will still contain a number of neighbouring voxels that have less than 75% grey matter (these are classified by the programme as empty voxels); as a consequence, it is possible that regional atrophy of grey matter rather than intrinsic abnormality per se could lead to the observed MTR abnormality. To evaluate this potential confounding effect, we determined the actual percentage of grey matter in the regions that were classified as different on MTR. This additional analysis revealed no evidence for focal grey matter atrophy in the visual cortex of patients versus controls. It, therefore, seems most likely that the MTR abnormalities observed in visual cortex reflect intrinsic structural changes rather than the effects of atrophy or partial volume effects.
The MTR abnormalities were apparent in anterior regions of the visual cortex but not at the occipital pole. The explanation for this anatomical location within the visual cortex is uncertain; however, resolution of the MTR sequence was limited (5 mm slice thickness) and the use of higher resolution 3D MTR sequences in future studies may provide more precise information on the location of visual cortex abnormalities.
The MT effect is based on the interactions between protons in a relatively free environment and those where motion is restricted. The MTR measures the capacity of the macromolecules in the CNS to exchange magnetization with the surrounding water molecules and provides a quantitative measure of macromolecule structural characteristics (Tofts et al., 2003). MTR has proven sensitive in detecting morphological changes in normal appearing tissues in multiple sclerosis and the pathological features associated with decreased MTR have been investigated. In post-mortem studies of multiple sclerosis brain, MTR has been related to both myelin content (Barkhof et al., 2003) and axonal density (van Waesberghe et al., 1999). Recently, Schmierer et al. studied these two pathological features—which are correlated with each other—and found that MTR was related more to the myelin content in white matter of multiple sclerosis patients (Schmierer et al., 2004). No data are available concerning the pathological substrate of GM MTR decrease in multiple sclerosis although demyelinating lesions—which are frequently seen in grey matter (Kidd et al., 1999; Peterson et al., 2001)—are likely to contribute.
Potential mechanisms for MTR decrease in visual cortex
In patients, the selectivity of the GM MTR decrease in the visual cortex argues against an effect of demyelinating cortical lesions per se—as occur in multiple sclerosis (Kidd et al., 1999; Peterson et al., 2001)—because such lesions should be more widely distributed in cortical grey matter. The selective location in the visual cortex suggests a more specific link with the symptomatic inflammatory demyelinating optic nerve lesion, and implies a trans-synaptic pathogenic mechanism.
Trans-synaptic degeneration or dystrophy
A possible mechanism for the MTR decrease is the effect of anterograde trans-synaptic changes secondary to axonal transection in the acute lesions of the optic nerve. Damage to or degeneration of the retinal ganglion cell axons might induce dystrophic changes in the neuron connecting the lateral geniculate nucleus and the striate cortex. Using MR diffusion tractography-based group mapping, Ciccarelli et al. have demonstrated reduced connectivity in optic radiations in seven patients one year after an attack of optic neuritis (Ciccarelli et al., 2005) and proposed that these changes may be related to trans-synaptic degeneration secondary to optic nerve damage and loss of afferent axons in the lateral geniculate nucleus. Trans-synaptic changes in the lateral geniculate body as a result of optic nerve lesions have been described in animal models (Madigan et al., 1996) and in patients with multiple sclerosis (Goldby, 1957; Evangelou et al., 2001). Evangelou et al. demonstrated a relatively selective atrophy of the smaller neurons of the parvocellular layer in both lateral geniculate nuclei in patients with multiple sclerosis (Evangelou et al., 2001). In the present study, the lack of significant lateral geniculate nucleus MTR changes may be explained by several methodological limitations: given the small size of this deep grey matter structure, the slice thickness of the scans acquired (5 mm) and the characteristics of the segmentation technique (using voxel intensities and a priori knowledge procedures), it is conceivable that the lateral geniculate nucleus was not included in the MTR grey matter maps during the segmentation process.
Limited data is available concerning the impact of lateral geniculate nucleus neuronal loss on neurons in the visual cortex. Non-human primate studies of chronic glaucoma have suggested that damage to the retinal ganglion cells due to intra-ocular pressure causes degenerative changes in the lateral geniculate nucleus and primary visual cortex (Yucel et al., 2003). In the former, diffuse neuronal loss and neuronal shrinkage proportionate to retinal ganglion cells loss have been described. In the visual cortex, altered cytochrome oxidase activity indicating reduction of the metabolic activity has been observed in recipient cortical layers (Crawford et al., 2000). In humans, two post-mortem studies (Wilson, 1963; Adams et al., 1966) and one in vivo magnetization transfer and diffusion tensor MRI study (Inglese et al., 2001) showed no abnormality in the optic radiation and calcarine cortex of patients with Leber's hereditary optic neuropathy, a condition in which there is axonal loss in the optic nerve. In contrast, two studies using phosphorus MRS (31P-MRS) showed altered mitochondrial energy metabolism in the white matter of the occipital lobe from patients with the same condition (Cortelli et al., 1991; Barbiroli et al., 1995). Using MRI, Kitajima et al. reported calcarine atrophy in patients suffering from retinal degeneration, suggesting that trans-synaptic degenerative changes may occur in the calcarine area after retinal degeneration (Kitajima et al., 1997).
Although previous findings are not always consistent, there seems to be reasonable evidence to suggest that trans-synaptic dystrophy or degeneration might occur following axonal degeneration in the optic nerve such that it causes morphological changes in the visual cortex in patients with optic neuritis. Although small, the correlation between decreased visual acuity (at baseline and 3-month follow-up) and MTR decrease in the visual cortex argues in favour of this interpretation. Visual acuity—especially at the 3 month follow-up time point—probably reflects the degree of optic nerve damage including axonal loss (Trip et al., 2005), which in turn may induce the distant morphological changes in the visual cortex.
The lack of significant atrophy in the visual cortex in these patients suggests that the local structural changes do not include a significant degree of neuronal loss, although it is conceivable that atrophy might only appear after a longer period of follow-up from the episode of optic neuritis if chronic neuronal dysfunction were to eventually culminate in neuronal death.
Morphological changes due to plasticity
Using fMRI in patients affected previously by optic neuritis, several studies have suggested evidence for plasticity in the visual cortex (Werring et al., 2000; Toosy et al., 2002; Toosy et al., 2005). A recent study correlating structural imaging of the symptomatic optic nerve lesion with visual fMRI response suggested an adaptive role for cortical reorganization within extra-striate visual areas early after optic neuritis (Toosy et al., 2005). It seems plausible that plasticity mechanisms—such as unmasking of previously present but functionally inactive connections, growth of new connections (collateral sprouting) and increased cell genesis—could potentially result in morphological changes that cause a subtle decrease in MTR.
Optic radiation abnormalities
Just over 50% of optic neuritis subjects had one or more T2 lesions in the optic radiations, which potentially might have led to structural changes in the visual cortex by anterograde trans-synaptic changes secondary to axonal transection in the radiation lesions. However, there was no difference in visual cortex MTR between those subjects with and without radiation lesions and the subgroup without radiation lesions had reduced visual cortex MTR compared to controls. It is therefore unlikely that the radiation lesions are the main cause for the visual cortex abnormalities observed. Moreover, although optic radiation MTR was reduced in patients versus controls, there was no correlation between patients' optic radiation and GM MTR. Quantitatively small changes in MTR, such as were seen in the optic radiation, could be caused by several processes that are known to affect the normal appearing white matter (NAWM) in multiple sclerosis—these include inflammation, glial proliferation and axonal loss. The lack of relationship with visual cortex MTR changes may indicate an independence of the MTR-detected processes occurring in optic radiation and visual cortex, or a limited sensitivity in evaluating correlations where there are only small quantitative changes.
Other observations
In patients fulfilling McDonald's criteria for multiple sclerosis, the more widespread GM MTR changes observed could be related to the higher T2 lesion load compared to patients not fulfilling McDonald's criteria (see Table 1). Thus, in patients fulfilling the McDonald criteria, axonal transection in the more numerous white matter lesions located in the periventricular areas may induce Wallerian or anterograde degeneration extending to grey matter regions with particularly extensive output and input connections, such as the hippocampus, the superior temporal gyrus, the cerebellum and the lenticular nucleus. MTR decreases in the lenticular nucleus have been previously described in patients presenting with a CIS and fulfilling the McDonald imaging criteria for multiple sclerosis (Audoin et al., 2004). Focal grey matter atrophy per se may also have contributed to the MTR decrease observed in the right lenticular nucleus. Another potential cause for more extensive GM MTR abnormality might be that cortical lesions are more common in patients with a larger white matter lesion load.
In patients with no visible T2 lesions the lack of any significant GM MTR changes may be related to the small size of this group (n = 16) or to a smaller degree of axonal transection in the symptomatic optic nerve (and consequently less change in the visual cortex).
Conclusion
The finding of a specific MTR abnormality in visual cortex in optic neuritis patients suggests that in addition to cortical grey matter demyelinating lesions and the direct effect of retrograde axonal degeneration from white matter lesions to the corresponding neuronal cell body, trans-synaptic morphological changes contribute to the abnormalities of MTR seen in the normal appearing grey matter of multiple sclerosis. Further clarification of the relative importance of these multiple processes should become possible when better methods emerge for detecting cortical lesions, and with the application of diffusion tractography to delineate the anatomical link between white matter lesions and the relevant cortical or deep grey matter region.
The NMR Research Unit is supported by the Multiple Sclerosis Society of Great Britain and Northern Ireland. Dr Audoin is supported by the French Association for Research on Multiple Sclerosis (A.R.S.E.P.). We thank Drs Catherine Dalton and Peter Brex for assistance with patient recruitment and Dr Jean Philippe Ranjeva for methodological advice.
References
Adams JH, Blackwood W, Wilson J. Further clinical and pathological observations on Leber's optic atrophy.
Audoin B, Ranjeva JP, My Van AD, Ibarrola D, Malikova I, Confort-Gouny S, et al. Voxel-based analysis of MTR images: a method to locate gray matter abnormalities in patients at the earliest stage of multiple sclerosis.
Audoin B, Van Au Duong M, Ranjeva JP, Ibarrola D, Malikova I, Confort-Gouny S, et al. Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis.
Barbiroli B, Montagna P, Cortelli P, Iotti S, Lodi R, Barboni P, et al. Defective brain and muscle energy metabolism shown by in vivo 31P magnetic resonance spectroscopy in nonaffected carriers of 11778 mtDNA mutation.
Barker GJ, Tofts PS, Gass A. An interleaved sequence for accurate and reproducible clinical measurement of magnetization transfer ratio.
Barkhof F, Bruck W, De Groot CJ, Bergers E, Hulshof S, Geurts J, et al. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance.
Bjartmar C, Trapp BD. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences.
Bozzali M, Cercignani M, Sormani MP, Comi G, Filippi M. Quantification of brain gray matter damage in different multiple sclerosis phenotypes by use of diffusion tensor MR imaging.
Brex PA, Gomez-Anson B, Parker GJ, Molyneux PD, Miszkiel KA, Barker GJ, et al. Proton MR spectroscopy in clinically isolated syndromes suggestive of multiple sclerosis.
Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis.
Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, Barker GJ, Parker GJ, Thompson AJ, et al. Investigation of multiple sclerosis normal-appearing brain using diffusion tensor MRI with clinical correlations.
Ciccarelli O, Toosy AT, Hickman SJ, Parker GJ, Wheeler-Kingshott CA, Miller DH, et al. Optic radiation changes after optic neuritis detected by tractography-based group mapping.
Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM. Thalamic neurodegeneration in multiple sclerosis.
Cortelli P, Montagna P, Avoni P, Sangiorgi S, Bresolin N, Moggio M, et al. Leber's hereditary optic neuropathy: genetic, biochemical, and phosphorus magnetic resonance spectroscopy study in an Italian family.
Crawford ML, Harwerth RS, Smith EL 3rd, Shen F, Carter-Dawson L. Glaucoma in primates: cytochrome oxidase reactivity in parvo- and magnocellular pathways.
Dalton CM, Brex PA, Jenkins R, Fox NC, Miszkiel KA, Crum WR, et al. Progressive ventricular enlargement in patients with clinically isolated syndromes is associated with the early development of multiple sclerosis.
Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, et al. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes.
Davies GR, Altmann DR, Hadjiprocopis A, Rashid W, Chard DT, Griffin CM, et al. Increasing normal-appearing grey and white matter magnetisation transfer ratio abnormality in early relapsing-remitting multiple sclerosis.
De Stefano N, Matthews PM, Filippi M, Agosta F, De Luca M, Bartolozzi ML, et al. Evidence of early cortical atrophy in multiple sclerosis: relevance to white matter changes and disability.
Dehmeshki J, Chard DT, Leary SM, Watt HC, Silver NC, Tofts PS, et al. The normal appearing grey matter in primary progressive multiple sclerosis: a magnetisation transfer imaging study.
Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis.
Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis.
Fernando K, Tozer DJ, Miszkiel KM, Gordon RM, Swanton JK, Dalton CM, et al. Magnetization transfer histograms in clinically isolated syndromes suggestice of multiple sclerosis.
Filippi M. Multiple sclerosis: a white matter disease with associated gray matter damage.
Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, et al. Analysis of fMRI time-series revisited.
Ge Y, Grossman RI, Udupa JK, Babb JS, Nyul LG, Kolson DL. Brain atrophy in relapsing-remitting multiple sclerosis: fractional volumetric analysis of gray matter and white matter.
Goldby F. A note on transneuronal atrophy in the human lateral geniculate body.
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains.
Grimaud J, Lai M, Thorpe J, Adeleine P, Wang L, Barker GJ, et al. Quantification of MRI lesion load in multiple sclerosis: a comparison of three computer-assisted techniques.
Iannucci G, Tortorella C, Rovaris M, Sormani MP, Comi G, Filippi M. Prognostic value of MR and magnetization transfer imaging findings in patients with clinically isolated syndromes suggestive of multiple sclerosis at presentation.
Inglese M, Rovaris M, Bianchi S, Comi G, Filippi M. Magnetization transfer and diffusion tensor MR imaging of the optic radiations and calcarine cortex from patients with Leber's hereditary optic neuropathy.
Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis.
Kitajima M, Korogi Y, Hirai T, Hamatake S, Ikushima I, Sugahara T, et al. MR changes in the calcarine area resulting from retinal degeneration.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS).
Madigan MC, Rao NS, Tenhula WN, Sadun AA. Preliminary morphometric study of tumor necrosis factor-alpha (TNF alpha)-induced rabbit optic neuropathy.
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis.
Miller DH, Thompson AJ, Filippi M. Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis.
Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions.
Quarantelli M, Ciarmiello A, Morra VB, Orefice G, Larobina M, Lanzillo R, et al. Brain tissue volume changes in relapsing-remitting multiple sclerosis: correlation with lesion load.
Ranjeva JP, Audoin B, Duong MV, Ibarrola D, Confort-Gouny S, Malikova I, et al. Local tissue damage assessed with statistical mapping analysis of brain magnetization transfer ratio: relationship with functional status of patients in the earliest stage of multiple sclerosis.
Rovaris M, Filippi M, Minicucci L, Iannucci G, Santuccio G, Possa F, et al. Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis.
Rovaris M, Bozzali M, Iannucci G, Ghezzi A, Caputo D, Montanari E, et al. Assessment of normal-appearing white and gray matter in patients with primary progressive multiple sclerosis: a diffusion-tensor magnetic resonance imaging study.
Rugg-Gunn FJ, Eriksson SH, Boulby PA, Symms MR, Barker GJ, Duncan JS. Magnetization transfer imaging in focal epilepsy.
Sailer M, Fischl B, Salat D, Tempelmann C, Schonfeld MA, Busa E, et al. Focal thinning of the cerebral cortex in multiple sclerosis.
Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain.
Sharma R, Narayana PA, Wolinsky JS. Grey matter abnormalities in multiple sclerosis: proton magnetic resonance spectroscopic imaging.
Simon JH, Kinkel RP, Jacobs L, Bub L, Simonian N. A Wallerian degeneration pattern in patients at risk for multiple sclerosis.
Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc.,
Tiberio M, Chard DT, Altmann DR, Davies G, Griffin CM, Rashid W, et al. Gray and white matter volume changes in early RRMS: a 2-year longitudinal study.
Tofts PS, Steens SCA, van Buchem MA. MT: magnetization transfer. In Tofts PS, editor. Quantitative MRI of the brain. Measuring changes caused by disease. Chichester: John Wiley; 2003. p. 257–98.
Toosy AT, Werring DJ, Bullmore ET, Plant GT, Barker GJ, Miller DH, et al. Functional magnetic resonance imaging of the cortical response to photic stimulation in humans following optic neuritis recovery.
Toosy AT, Hickman SJ, Miszkiel KA, Jones SJ, Plant GT, Altmann DR, et al. Adaptive cortical plasticity in higher visual areas after acute optic neuritis.
Traboulsee A, Dehmeshki J, Brex PA, Dalton CM, Chard D, Barker GJ, et al. Normal-appearing brain tissue MTR histograms in clinically isolated syndromes suggestive of multiple sclerosis.
Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis.
van Waesberghe JH, Kamphorst W, De Groot CJ, van Walderveen MA, Castelijns JA, Ravid R, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability.
Werring DJ, Bullmore ET, Toosy AT, Miller DH, Barker GJ, MacManus DG, et al. Recovery from optic neuritis is associated with a change in the distribution of cerebral response to visual stimulation: a functional magnetic resonance imaging study.
Wilson J. Leber's hereditary optic atrophy: some clinical and aetiological considerations.
Wylezinska M, Cifelli A, Jezzard P, Palace J, Alecci M, Matthews PM. Thalamic neurodegeneration in relapsing-remitting multiple sclerosis.
Author notes
1NMR Research Unit, Departments of 2Neuroinflammation and 3Headache, Rehabilitation and Brain Injury, Institute of Neurology, University College London, 4Neuro-ophthalmology Service, Moorfields Eye Hospital, London, UK and 5Centre de Résonance Magnétique Biologique et Médicale (CRMBM) and Service de Neurologie, Hôpital de la Timone, Marseille, France