Brain magnetic resonance imaging (MRI) features of chronic hepatic encephalopathy in liver cirrhosis include diffuse cerebral atrophy symmetric high signals in the basal ganglia namely the globus pallidus bilaterally on T1 weighted images, with no corresponding high signals on the T2 weighted images.1 It has been postulated that the globus pallidus high signals are related to manganese deposition secondary to reduced hepatobiliary excretion associated with liver cirrhosis.1 However, it is unclear whether the presence of T1-globus palldus high signals is a manifestation of hepatic encephalopathy, or if it occurs secondary to liver cirrhosis, cholestasis or results from the porto-systemic shunt associated with chronic liver disease.2 Additional MRI findings include white matter abnormalities related to central nervous system (CNS) accumulation of ammonia. The white matter abnormalities can be detected with magnetisation transfer, fast fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI) techniques on MRI imaging.3 These MR imaging abnormalities usually return to normal upon normalization of the hepatic function.3 Recent reports of uncommon MRI findings in acute hepatic encephalopathy with hyperammonemia are described resembling hypoxic-ischemic brain injury.4 These findings include diffuse cortical necrosis secondary to hyperammonemia with restricted diffusion on MRI in the insular and the cingulate cortices, subcortical white matter, bilateral thalami, and brainstem.5 These abnormalities appear as high signals on FLAIR & T2 weighted images in the corresponding regions.4,5 In this report, we describe unusual brain MRI features of a patient of liver cirrhosis in acute hepatic encephalopathy and hyperammonemia with bilateral symmetrical thalamic lesions that shows persistence diffusion restriction on DWI and high signals on FLAIR sequences in addition to MRI features of the chronic hepatic disease. The symmetrical and persistence diffusion restriction of both thalami is unusual in a hemodynamically stable patient.
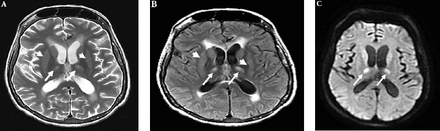
A 61-year-old lady with diabetes mellitus, primary hypothyroidism old ischemic stroke and post-stroke epilepsy was diagnosed with decompensated cryptogenic liver cirrhosis child class C and MELD score 24 admitted to our hospital for evaluation for liver transplantation. She had several previous admissions to the hospital for the treatment of recurrent episodes of hepatic encephalopathy and had 2 sessions of band ligation of esophageal varices. Clinical examination revealed drowsy and confused patient with bilateral flapping tremors of the hands. In addition, she had ascites with other signs of decompensated liver cirrhosis. Laboratory investigations showed anemia with hemoglobin of 7g/dl (normal value is 11.5-16.6), thrombocytopenia of 35 10”9/L(normal value is 150-450), normal transaminases and total bilirubin, mildly raised alkaline phosphatase of 133 U/L (normal value is 35-104), and decreased albumin at 24 g/L (normal value is 35-52), normal total cholesterol, fasting blood glucose and renal function. The plasma ammonia level on admission was twice the normal level 186 µmol/L; (normal value is 23-76). Other laboratory investigations include normal prothrombin time (PT) and normalized international ratio (INR), hepatitis A, B, C, E viruses, cytomegalovirus, Epstein Barr virus and human immunodeficiency viruses (HIV1 and HIV2) screen was negative. Blood screening for autoimmune disorders and connective tissue diseases was negative. Chest roentgenogram showed mild bilateral pleural effusion. Abdominal echosonography and computed tomography (CT) revealed atrophic cirrhotic liver, splenomegaly and ascites. Electroencephlogram (EEG) showed a slow background with bilateral triphasic waves consistent with hepatic encephalopathy. Brain magnetic resonance imaging (MRI) showed bilateral symmetrical high signal thalamic lesions and bright signals of the basal ganglia bilaterally on T2 weighted (Figure 1A) and FLAIR images (Figure 1B). The thalamic lesions showed diffusion restriction with bilateral high signal intensities in the central aspect of both thalami on DWI (Figure 1C). The Apparent diffusion coefficient (ADC) map images showed bilateral thalamic faint hypodensities in both thalami indicating some Oedema. Magnetic resonance angiogram (MRA) and magnetic resonance venogram (MRV) were normal. Magnetic resonance brain imaging performed in a previous admission one year before the recent admission for evaluation of her epilepsy and ischemic stroke failed to show any thalamic lesions seen on the recent brain MRI. According to the history and the results of EEG, in combination with laboratory and radiological data, acute hepatic encephalopathy with hyperammonemia was confirmed. Clinically the patient improved his level of consciousness and became oriented after the treatment of hyperammonemia and the resolution of acute hepatic encephalopathy. The plasma ammonia level returned to a normal level concurrently with the clinical improvement. Follow-up brain MRI after 2 weeks of treatment showed the persistence of the bilateral symmetrical thalamic lesions with diffusion restriction on DWI despite clinical recovery, a radiological feature not previously seen in hepatic encephalopathy.
The MRI brain at presentation of the patient with acute hepatic encephalopathy. A) T2 Axial image shows bilateral high signal intensities involving both thalami (white arrows) & basal ganglia (white arrow heads). B) FLAIR Axial image shows bilateral high signal intensities involving both thalami (white arrows) & basal ganglia (white arrow heads). C) Diffusion weighted axial image shows the thalamic lesions with diffusion restriction and bilateral high signal intensities in the central aspect of both thalami (white arrows).
Thalamic lesions on brain MRI are seen in several disorders that include ischemic vascular diseases, metabolic disorders such as Fabry disease, osmotic demyelinating syndrome, Wernicke encephalopathy, inherited metabolic disorders. Neuroinflammatory diseases such as Behcet disease and Sjogren’s syndrome, demyelinating disorders such as acute disseminated encephalomyelitis, trauma, tumors such as glioblastoma multiforme and gliomatosis cerebri, at times in infections such as encephalitis and brain abscess and rarely in multiple sclerosis and reversible posterior leukoencephalopathy syndrome.1 Bilateral thalamic lesions on brain MRI are uncommon in chronic liver disease and reported in few cases of acute hepatic encephalopathy with hyperammonemia resembling hypoxic-ischemic brain injury with restricted diffusion on DWI and high signals on FLAIR and T2 weighted MRI images.1 In reported cases the brain MRI restricted diffusion on DWI images could also be seen in the insular and the cingulate cortices, subcortical white matter and brainstem.4 The described thalamic and other lesions were reversible in some reports with reduction of the plasma ammonia level which corresponded to the extent of the MRI abnormality.1,2,3 In our case of the chronic liver disease with cirrhosis presenting with acute hepatic encephalopathy and hyperammonemia the thalamic lesions on DWI images, FLAIR and T2 weighted sequences appeared in the brain MRI performed in the recent admission while the patient was symptomatic with hyperammonemia. Previous brain MRI brain of our patient showed only features of chronic liver disease confirming the the association of thalamic lesions with restricted diffusion on DWI and high signals on FLAIR images with acute hepatic encephalopathy with hyperammonemia reported in the literature.3 The thalamic lesions with the same brain MRI features persisted despite normalization of the plasma ammonia level; a fact possibly related to the short interval of the follow-up MRI brain images after treatment of hyperammonemia and follow-up brain MRI in 6 months is planned to assess the reversibility of the thalamic lesions after normalization of the hyperammonemia. Limited studies available in the literature to determine the reversibility of these lesions, and the correlation between the severity of the MR imaging involvement on FLAIR or DWI images with the plasma ammonia level and clinical outcome.3 Available brain MRI data suggest that FLAIR and DWI images abnormalities in the thalami occur in 85% and 70% of respected MRI images and diffuse cortical involvement in 30%, and 25% with relatively strong correlation of FLAIR and DWI images with higher plasma ammonia level, and of plasma ammonia level with the clinical outcome. The FLAIR and DWI images severity correlated moderately with the clinical outcome. Diffuse cortical involvement has a higher association with worse neurologic sequelae but can be reversible and the duration of those abnormal signals on brain MRI before reversibility is variable.1,4 As a result of raised CNS ammonia level, the CNS glutamine rises subsequently and leads to acute hepatic encephalopathy. The CNS glutamine accumulation results in astrocytic swelling, cytotoxic oxidative and nitrosative damage, and disruption of metabolism of glucose, defective gamma-aminobutyric acid GABA synthesis, and disrupted blood-brain barrier permeability.5 As a result of the disturbed neuronal function and raised brain glutamine clinical manifestations of acute hepatic encephalopathy appear with cerebral oedema and depressed consciousness.5 Our report confirms the usefulness of brain MRI in the evaluation of acute hepatic encephalopathy. The T1 weighted images shows the classical globus pallidus high signals in chronic liver disease. FLAIR and DWI images may show the characteristic thalamic lesions with or without other features of acute hyperammonemia and therefore enables early diagnosis, appropriate management and assess possible prognosis of patients.
In conclusion, this report alerts the reader of the unusual radiological findings of hyperammonemia in acute hepatic encephalopathy associated with bilateral symmetrical thalamic lesions with diffusion restriction on brain MRI resembling hypoxic-ischemic brain injury which is irreversible and associated with poor neurological outcome.
- Received December 19, 2017.
- Accepted January 24, 2018.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.