Abstract
Familial idiopathic basal ganglia calcification (Fahr’s disease) is a rare neurodegenerative disorder characterized by symmetrical and bilateral calcification of the basal ganglia. Calcifications may also occur in other brain regions such as dentate nucleus, thalamus, and cerebral cortex. Both familial and non-familial cases of Fahr’s disease have been reported, predominantly with autosomal-dominant fashion. The disease has a wide range of clinical presentations, predominantly with neuropsychiatric features and movement disorders. Psychiatric features reported in the literature include: cognitive impairment, depression, hallucinations, delusions, manic symptoms, anxiety, schizophrenia-like psychosis, and personality change. Other clinical features include: Parkinsonism, ataxia, headache, seizures, vertigo, stroke-like events, orthostatic hypotension, tremor, dysarthria, and paresis. Fahr’s disease should be considered in the differential diagnosis of psychiatric symptoms, particularly when associated with movement disorder. The disease should be differentiated from other conditions that can cause intracranial calcification. No specific treatment is currently available. Further research is needed to bridge the gap existing in our current knowledge of the prevalence, etiology, symptoms, and treatment of Fahr’s disease.
Fahr’s disease, also known as familial idiopathic basal ganglia calcification, is a rare neurodegenerative disorder that is characterized by calcifications in the basal ganglia and other brain regions. Basal ganglia calcification can be asymptomatic or can be associated with neuropsychiatric and motor symptoms. Fahr was a German neurologist who reported, in 1930, a case of an 81-year-old patient with symptoms of dementia, fever, and immobility, and brain autopsy examination showed evidence of calcification in the striatum.1 The term Fahr’s disease refers to cases of idiopathic basal ganglia calcification. The term Fahr’s syndrome has also been used in the literature to indicate cases of secondary basal ganglia calcification. Fahr’s disease has variable clinical manifestations and clinical definitions. It can be clinically defined as bilateral calcifications in the presence of neuropsychiatric and extra-pyramidal disorders, while calcium and phosphorus metabolism are normal. Fahr’s disease can also be defined clinically by the presence of seizures, rigidity, and dementia with characteristic calcification of the basal ganglia.1,2 This review aims to explore neuropsychiatric presentations in patients with idiopathic basal ganglia calcification. It is good practice to rule out possible organic etiologies for patients with psychiatric symptoms. If an organic etiology is identified, then the diagnosis will be organic psychiatric disorder, which may have symptoms similar to those of the functional type such as schizophrenia. In such a situation, the term schizophrenia-like should be given. The same rule may be applied for other similar presentations such as mood disorder and cognitive dysfunction.
Etiology and pathophysiology
Both familial and nonfamilial cases of Fahr’s disease have been reported, predominantly with an autosomal-dominant fashion.3,4 A whole-genome scan, performed for 24 members of a multigenerational family with dominantly inherited idiopathic basal ganglia calcification, revealed a locus for Fahr’s disease on chromosome 14q.5 The link between calcium homeostasis and a compromised blood brain barrier has been hypothesized as an etiological factor for both childhood psychiatric disorders (such as Asperger’s syndrome and attention deficit hyperactivity disorder [ADHD]) and adulthood psychiatric disorders (such as schizophrenia, mood disorders, and anxiety disorders). The hypothesis is based on the findings that psychiatric conditions may be associated with calcium dysregulation, calcium signaling, and altered calcium homeostasis. In adult patients with schizophrenia and bipolar disorder, there is an increase of neuronal calcium sensor-1 in the dorsolateral prefrontal cortex of the brain.6 There is no clear explanation for the occurrence of calcification in patients with Fahr’s disease. Possible causes of calcification are metastatic deposition, being secondary to local disruption of blood brain barrier, or this may be due to a disorder of neuronal calcium metabolism.7 Homocarnosine, a CNS-specific peptide, was found to be increased 2-fold in the CSF analysis of patients with Fahr’s disease; in sporadic cases, homocarnosine was not detectable and the histidine level was found low.8 High levels of copper, zinc, iron, and magnesium have also been detected in the CSF of some patients with Fahr’s disease.9 Evaluation of regional cerebral blood flow to the areas of calcification revealed markedly decreased perfusion to the basal ganglia bilaterally, and decreased perfusion to the cerebral cortex.10 Reduction of glucose metabolism in the basal ganglia and the frontal brain region has been reported in patients with Fahr’s disease, which may indicate a disruption of frontostriatal circuits.11 Gross pathologic brain examination has shown granular material and solid nodules accumulating in the striatum, internal capsule, white matter, and cerebellum. Histologically, concentric calcium deposits were seen within small and medium-sized arterial walls. Droplet calcifications can be observed along capillaries. Diffuse gliosis may occur surrounding the large calcium deposits, and ischemic changes may exist in the basal ganglia, cortical areas, and sub-cortical regions.12
Basal ganglia and neuropsychiatric symptoms
The basal ganglia are a group of subcortical nuclei that have been linked to control of movement. The primary structures of the basal ganglia are the striatum (including the caudate nucleus, putamen, and nucleus accumbens), the globus pallidus, substantia nigra, and subthalamic nucleus. The basal ganglia and cerebellum were traditionally thought to provide output only to the primary motor cortex via the thalamus. This view has been challenged, and the cerebellar output may also target the prefrontal cortex areas that are involved in language and cognitive functions as well as limbic functions.13 The basal ganglia are divided into dorsal and ventral systems. The dorsal striatum is associated with motor and cognitive functions, while the ventral striatum is associated with motivational functions. The ventral striatum is composed of parts of the basal ganglia that are closest to limbic structures including the nucleus accumbens.14 The ventral striatum can be divided into a central core surrounded by a shell, which has rich dopaminergic innervations arising from the ventral tegmental area and dense innervations from the basolateral complex of the amygdala.15 The functions of the basal ganglia and the cerebellum may be integrated across motor and non-motor domains. Both the motor and non-motor domains of the dentate nucleus of the cerebellum provide disynaptic inputs to the basal ganglia. Also, the motor and non-motor domains of the substantia nigra provide disynaptic inputs to the cerebellar cortex. These interactions may have clinical implications for neuropsychiatric disorders such as schizophrenia, Tourette’s syndrome, autistic disorder, attention deficit disorder, and drug dependence.13 In the basal ganglia, there is a complexity of neurotransmitter distribution, interaction, and function involving dopamine, serotonin, acetylcholine, excitatory amino acids, GABA, nitric oxide, neuro-peptides, and adenosine.15 The basal ganglia are thought to be involved in several functions including motor learning, sequencing, movements, attentional allocation, working memory, and implicit memory. These operations may have roles in the acquisition of automatically-performed behaviors as well as in enhancing the efficiency of higher order processors like those involved in working memory, and reward processes.16,17 Clinically, interactions between dopamine and acetylcholine are applied in Parkinson’s disease, and interactions between dopamine and serotonin play a role in the motor side effects of psychotropic medications. The basal ganglia may be involved in generating neuropsychiatric symptoms in major psychiatric disorders such as obsessive-compulsive disorder, schizophrenia, depression, and addiction. Several basal ganglia disorders can have mental and cognitive manifestations, which occur mainly due to abnormalities in the basal ganglia, although the pathophysiology of these conditions can also be due to pathology outside the basal ganglia such as the thalamus, the frontal cortex, or aminergic nuclei. Examples of basal ganglia disorders that may have neuropsychiatric symptoms include: Parkinson’s disease, Wilson’s disease, progressive supranuclear palsy, Gilles de la Tourette’s syndrome, and Fahr’s disease.15 A recent review suggests association between depression and Parkinsonian symptoms; personality changes and caudate or putamen disease; psychosis and caudate disease. Dementia and manic symptoms could be associated with caudate and pallidal diseases; and compulsions may have a relationship with pallidal disease.18
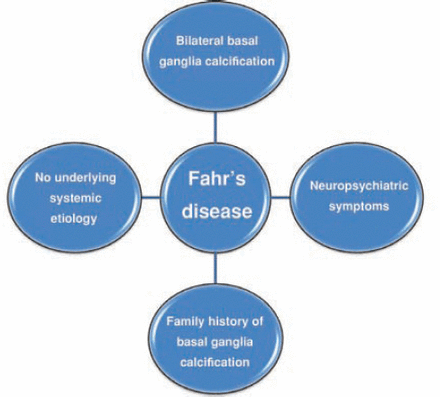
Clinical features of Fahr’s disease
Clinical manifestations of Fahr’s disease are reported in the literature either as individual case reports or as family reports due to the clinical rarity of the disease.19 Clinical features are summarized in Table 1, and the criteria for diagnosis are shown in Figure 1. The disease has an insidious onset and is usually described in middle-aged patients presenting with various neurological and psychiatric symptoms. Bilateral symmetric calcification can occur in a wide range of conditions. It can be asymptomatic or can be associated with variable clinical presentations, such as neurological conditions or pseudo-pseudohypoparathyroidism. The criteria for the diagnosis of Fahr’s disease include:1,20 evidence for bilateral basal ganglia calcification; progressive neurological or neuropsychiatric manifestations; the onset of symptoms usually occurs in the fourth or fifth decade (earlier onset can also occur); there is no evidence from biochemical abnormalities and clinical features that suggest presence of a mitochondrial or metabolic disease or other systemic disorders; the calcification is not due to infection, trauma, or toxic causes; and family history of basal ganglia calcification that is consistent with autosomal dominant inheritance. If there is a family history, the diagnosis can be made in the absence of one of the first 2 criteria. If the family history is negative, meeting the first 5 criteria is sufficient for the diagnosis of Fahr’s disease only if the calcifications are typical of Fahr’s disease.1,20 Calcification is most commonly reported in the globus pallidus. Additional reported sites of calcification include putamen, caudate nucleus, internal capsule, dentate nucleus, thalamus, cerebellum, and cerebral white matter.8 Several cases were diagnosed incidentally1,21-24 during routine assessment of the presenting psychiatric or somatic symptoms, which may raise questions on the rarity of the disease and may also suggest the possibility of an under-reporting of cases presenting with Fahr’s disease.
Clinical presentations of Fahr’s disease as reported in the literature.
Criteria for the diagnosis of familial idiopathic basal ganglia calcification (Fahr’s disease).
Psychiatric features
Psychiatric features are the initial presenting symptoms in around 40% of patients with basal ganglia calcifications. The common psychiatric features are cognitive deterioration, psychotic symptoms, and mood disorders.25 A retrospective study including 1942 patients with Fahr’s disease, aged between 20 and 96 years old, found depressive symptoms the most common psychiatric symptom in all age groups. Other common psychiatric features were anxiety, cognitive impairment, hallucinations, personality disorder, schizoid psychosis, and dementia.20 Organic affective symptoms were found more common in chronic cases of basal calcification than those with initial presentation, with depression being the most commonly reported mood disorder.25 Late-onset bipolar mood disorder has been reported. For example, Ghormode et al26 reported a 65-year-old male who developed his first episode of depression when he was 48 years old, which was followed by 4 manic episodes each lasting 2-4 months.26 Two patterns of psychotic features in Fahr’s disease have been reported in the literature: an early onset presentation with minimal movement disorder, and a late onset presentation associated with dementia and movement disorder.17 Some cases present with late onset paranoid delusions with associated mild cognitive impairment.22 Early onset schizophrenia-like psychosis with Fahr’s disease, presenting with auditory hallucinations, delusions, disorganized speech, perseveration, obsessions and inattention has been reported with calcifications involving the basal ganglia and dentate nucleus of the cerebellum.27 Cognitive symptoms in patients with Fahr’s disease include: dementia, delirium, and mental retardation. Cases of dementia with Fahr’s disease have been reported with neuropathological changes not due to Alzheimer’s disease or Pick’s disease.28 Some cases present with features of frontal lobe syndrome.29
Parkinsonism and movement disorders
Parkinsonism can present with both motor and non-motor symptoms. The motor symptoms include 4 cardinal features: bradykinesia, rest tremor, rigidity, and postural and gait impairment. The non-motor symptoms include neuropsychiatric features, dysautonomia (for example, orthostatic hypotension, sialorrhea, constipation), sleep disorders, sensory dysfunction, pain, and fatigue. The neuropsychiatric features in Parkinsonism include apathy, anxiety, panic attacks, mood disorders (particularly depression), hallucinations, illusions, and cognitive deterioration ranging from mild impairment to dementia.30 In a study combining 38 cases recruited through a registry and 61 cases reported in the literature, movement disorders were found the most common manifestations of Fahr’s disease accounting for 55% of the total symptomatic patients. Of the movement disorders, Parkinsonism was found in 57%, chorea in 19%, tremor in 8%, dystonia in 8%, athetosis in 5%, and orofacial dyskinesia in 3% of cases. Measurements of the total volume of calcification suggest a significantly greater amount of calcification in symptomatic patients compared with asymptomatic patients.31 It is important to consider the possibility of drug-induced Parkinsonism in patients with Fahr’s disease who received antipsychotic medications, and to differentiate it from Parkinsonian symptoms secondary to basal ganglia calcifications or Parkinson’s disease. Parkinsonian signs tend to present symmetrically with the presence of coarse postural tremor. In those whose symptoms are drug-induced, other disorders might be present, such as orolingual dyskinesias, tardive dystonia, or akathisia. The diagnosis of drug-induced Parkinsonism is likely if symptoms have emerged after the drug has been introduced. The symptoms of drug-induced Parkinsonism improve markedly or remit a few months after the drug has been withdrawn, but symptoms remain at least partially in those patients with a concomitant cause for Parkinsonism.32
Other symptoms
Other clinical findings in patients with Fahr’s disease are cranio-cerebral trauma, stroke, meningitis, encephalitis, brain tumors, cerebral aneurysm, arterio-venous malformation, subdural hematoma, and mastoiditis.20 Cases presenting with Fahr’s disease and disturbed calcium metabolism were found to be associated with idiopathic hypoparathyroidism, hyperparathyroidism, pseudo-hypoparathyroidism, and postoperative hypoparathyroidism.33 Patients presenting with parathyroid hormone deficiencies due to thyroidectomy have shown more severe mental deterioration.25
Differential diagnosis
The differential diagnosis of familial idiopathic basal ganglia calcification includes other conditions that can cause intracranial calcifications. The location of calcification and the clinical presentation are of vital importance in diagnosis. We have personal experience with patients presenting with brain calcifications who predominantly presented with psychiatric features. Two patients we encountered during our clinical practice were found to have basal ganglia calcifications on CT scan and brain MRI examinations, and one of the patients had calcification confined to the globus pallidus bilaterally. The psychiatric presentations included a long-standing history of poor attention and concentration, and short-term memory impairment, they had no abnormal findings on physical examination, and no underlying etiologies for calcifications were identified. Such presentations make the diagnosis more likely to be idiopathic basal ganglia calcification. We have also seen patients with calcifications in other brain regions. For example, a reported case of extensive falx-cerebri calcification presented with psychiatric symptoms and was found to have symptoms and signs suggestive of Gorlin-Goltz syndrome.34 Therefore, the location of calcifications and clinical features, as well as relevant investigations are important in differentiating types of calcifications, particularly those discovered incidentally during assessment of patients presenting with neuropsychiatric features, as there may be no other significant clinical features. Calcifications should be correlated with clinical findings for proper diagnosis of the underlying condition. Brain calcifications can be classified as extra-axial or intra-axial calcifications based on imaging findings. Table 2 provides a differential diagnosis of intra- and extra-axial brain calcifications.35 Calcifications can occur as physiologic, dystrophic, congenital, or vascular calcifications. Both acquired infections, and congenital infections can lead to intracranial calcifications. Examples of infections causing calcifications include TORCH infections (toxoplasmosis, other, rubella, cytomegalovirus, herpes simplex virus). Inflammatory lesions (such as sarcoidosis and tumors) may also lead to intracranial calcifications. Metabolic disorders that can affect calcium homeostasis can lead to calcifications that predominantly involve the basal ganglia. The basal ganglia calcifications are usually seen in the globus pallidus, the head of the caudate nucleus, and the putamen, and they commonly occur in middle-aged and elderly subjects. Brain calcifications are interpreted as incidental findings of no significance by some clinicians. However, individuals with brain calcifications, particularly those below the age of 30 years, should be carefully evaluated for underlying etiologies.36 Patients should be evaluated for underlying etiology so as to differentiate cases of idiopathic/genetic etiology from those who have calcifications secondary to other causes such as infections and metabolic/endocrine etiology. The following evaluations are suggested to identify possible etiologies:12 serum levels of alkaline phosphatase, calcitonin, and parathyroid hormone; metabolic, inflammatory, and infectious conditions; heavy metal concentrations; and CSF examination.
Differential diagnosis of brain calcifications.
Management
Treatment is currently symptomatic to improve the presenting neuropsychiatric symptoms. Treatment of underlying etiologies such as hypoparathyroidism has led to neuropsychiatric improvement, but there are no specific treatments that limit progression of calcification in the basal ganglia in Fahr’s disease, except for a theoretically unconfirmed report of using chelators with an antioxidant and calcium antagonist.37 Metal binding proteins and metal-chelating agents (such as ammonium tetrathiomolybdate, which is a Cu-chelating agent) have been theoretically suggested as one of the treatment options.9
Future research
Further research is needed to clarify the prevalence of the disease and the prevalence of psychiatric symptoms in basal ganglia calcification. The wide range of psychiatric and somatic symptoms raise questions whether there are subtypes of the disease depending on sites of calcification and the presenting symptoms. To improve our understanding of the disease and the associated psychiatric symptoms, psychiatrists need to carefully evaluate cases in which functional psychiatric diagnosis is questionable, and to consider neuroimaging investigations for early diagnosis of the disease and to report observations. Lauterbach and colleagues18 highlighted opportunities for further Fahr’s disease research including: calcium deposition, definition of heritability, mitochondrial disease, prevalence of psychiatric disorders and imaging correlates, longitudinal assessment of neuropsychiatric disorders, neuroprotective agents, pharmacologic interventions, treatment refractory conditions, and psychotherapy.
In conclusion, Fahr’s disease is a rare neurodegenerative disorder with characteristic bilateral symmetrical basal ganglia calcifications and dentate nucleus of the cerebellum. The disease should be considered in the differential diagnosis of psychiatric symptoms whether the symptoms are acute or chronic, and brain CT scan may help in early diagnosis. Further research is needed to bridge the gap existing in our current knowledge on the prevalence, etiology, symptoms, and treatment.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.