Clinically, central nervous system (CNS) fungal infections are difficult to diagnose and often have a deceptive presentation. In an immune-competent adult, an enhancing mass on magnetic resonance imaging (MRI) in the greater sphenoidal area has a high possibility of being a tumor like meningioma. An intracranial mass of fungal etiology in such patients is a highly unsuspected diagnosis. We report such a case eventually diagnosed as aspergilloma on biopsy. Instituting oral voriconazole resulted in complete resolution of the lesion. In the absence of predisposing factors or extracerebral foci of infection, direct CNS seeding by aspergillus is a poorly understood and rare occurrence. This case highlights the possible pathogenesis, challenges in diagnosis and eventually successful treatment protocol employed in an uncommon clinical scenario.
A 24-year-old male presented with complaints of repeated episodic holocranial, dull aching headache from last 4 months. The headache was moderate grade in intensity with no aggravating or relieving factors. This was associated occasionally with projectile vomiting and transient blurring of vision, which recovered immediately. Initially the patient complained of headache once in 5-6 days. The frequency of these episodes increased to once a day one month prior to presenting at our institute. The patient at this juncture also complained of lethargy and dizziness. There was no diurnal variation of symptoms or any history of loss of vision, fever, sore throat, sinusitis, seizures, or any neurological deficit. There was no history suggestive of diabetes mellitus, any chronic illness, present or previous drug intake including corticosteroids or anticancer drugs. He was conscious, alert but confused with unremarkable vital parameters (heart rate, blood pressure, respiratory rate and temperature). His mini-mental score was 22/30 (24-30). Neurological examination did not reveal any significant findings except bilateral early papilledema. The rest of the systemic examination was normal. All laboratory tests were within normal range. Chest, paranasal sinus and skull X-ray did not reveal anything significant. Computed tomography brain revealed a well-defined hyperdense sphenoidal lesion with perilesional edema, which showed homogenous enhancement on contrast (Figure 1a) with hydrocephalus. Magnetic resonance imaging showed a lobulated, extra-axial, homogenous contrast enhancing mass in the sphenoidal-basal area (Figure 1b). The lesion showed intermediate to high signals on the T2 weighted and fluid attenuated inversion recovery image with no abnormality in the underlying bone. T1 weighted sequence was heterogeneously low.
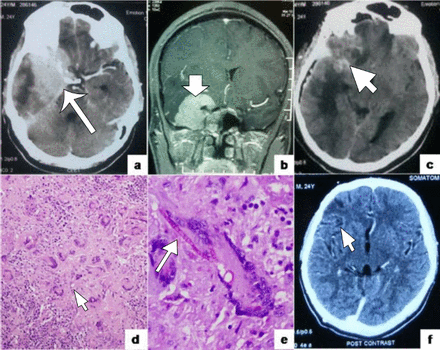
Diagnostic features of the present case a) Contrast enhanced computed tomography (CECT) showing a well defined enhancing basi-sphenoidal lesion (arrow) with adjoining parenchymal edema and hydrocephalus; b) Postcontrast MRI T1 weighted sequence (coronal view) showing an enhancing extra-axial dural based mass lesion (arrow); c) Immediate postoperative non contrast CT scan shows regression of the lesion and a small hyperdense focus corresponding to focal hemorrhage (arrow); d) Numerous histiocytic granulomas with lymphocytic cuffing, foreign body and langhans type giant cells. [Hematoxylin and Eosin, 20x]; e) Septate, acute angle branching fungal hyphae (arrow) within a giant cell highlighted by Periodic acid Schiff (PAS) stain. [Periodic acid Schiff, 40x]; f) Post contrast CT images taken after one year of follow up show complete resolution of the lesion and regression of surgery associated changes
A right pterional craniotomy with zygomatic osteotomy and near total excision of lesion was carried out. A small part attached to the internal carotid artery was left behind. Per-operative frozen sections suggested an infective pathology. Samples for culture and histopathological examination were sent. Immediate postoperative CT images showed regression of the lesion and focal hemorrhage secondary to surgery (Figure 1c). Post-operatively, the patient was stable and afebrile with maintained vital parameters. No neurological deficits were seen. The patient started recovering well, was asymptomatic and on examination showed resolving papilledema. The biopsy report revealed numerous granulomas with foreign body and few Langhans giant cells (Figure 1d) interspersed with eosinophils and lymphocytes. Fibrosis and areas of hemorrhage were noted. Periodic acid Schiff stain showed branching septate hyphae with yeast forms suggestive of aspergillus (Figure 1e). Evidence of thrombosis was not seen. Ziehl-Neelsen stain was negative for acid-fast bacilli. Mycotic culture report and microscopy, 3 weeks later confirmed the presence of aspergillus spp.
Oral voriconazole was started in a dose of 100 mg twice a day for 6 weeks. Investigations for tuberculosis and Human Immunodeficiency virus (HIV) were non contributory. The patient was kept under a vigilant follow up after discharge and was asked to come after every 2 weeks in the outpatient department (OPD). After 3 months, the symptoms recurred with the same intensity. Non-contrast computed tomography (NCCT) brain showed increased communicating hydrocephalus with bilateral papilledema. Ventriculo-peritoneal (VP) shunt was carried out through right occipital burr hole. Cerebrospinal fluid (CSF) pressure was raised and grossly clear. The CSF culture was sent to detect fungal infection but was non contributory. The patient has improved after that and is regularly being reviewed in the OPD every 2 months for the last one year. Currently, the patient is neurologically intact and is completely asymptomatic. Follow up CT images taken after one-year show a complete resolution of the lesion. (Figure 1f)
Intracranial fungal granuloma in immunocompetent adults is nearly always a rarity. With reference to human infection, fungi are mostly considered as opportunistic organisms, though they are commensals in the upper respiratory tract. These infections are commonly associated with humid climatic conditions or coastal environs. No such association was seen in our case. Invasive aspergillosis comprises 5% of intracranial fungal infection1 and may present as an intracranial space occupying lesion mimicking neoplasia as in this case. A PUBMED search for cases of craniocerebral aspergillomas mimicking meningioma in the immune competent confirmed the rarity of the present case.2 The anterior cranial fossa is commonly involved in such cases due to proximity of the nasal sinuses. Here the sinuses were clear and the lesion was centered at the basi-sphenoidal area ruling out contiguous spread. Hematogenous spread from a possible focus in the lungs or through intravenous drug usage did not apply in the current case. Our patient had localized disease and subtle constitutional symptoms such as weakness and lethargy. Repeated history taking and investigations failed to reveal an immunocompromised state. On the basis of the radiological findings the first diagnosis was sphenoid wing meningioma. Granulomatous etiology was a distant second as no signs or symptoms of inflammation were present. The aspergillotic intracranial granuloma shows hypointensity on T2-weighted images, possibly due to diamagnetic deoxyhemoglobin and ferritin3 which are by-products of chronic hemorrhage. Aspergillus is known to cause fibrosis, hemorrhage3 and subsequent iron deposition, which were seen in our case. However the diagnostic hypointense T2 was not found probably due to containment of disease by an intact immune system. A detailed clinical history and imaging to rule out nasal, paranasal, nasopharyngeal and pulmonary foci of infection is of paramount importance; however it may not yield fruit as in our case. In the absence of any valid etiology this “de novo aspergilloma” may be the result of inhalation of spores from the environment, activation of a latent infection during a transient period of low immunity, coupled with genetic predisposition. A recent animal model developed in immunocompetent rats has highlighted the persisting load of aspergillus in the brain vis-a-vis other organs.4 Why an aspergillotic fungal infection can bypass the nose and sinuses and preferentially afflict the CNS is a subject of further study. Larger studies by neurosurgical teams in collaboration with immunologists, pathologists and microbiologists are required to ascertain the exact cause of non-opportunistic fungal infection.
A word must be mentioned on the microbiological subtyping of the aspergillus isolated in this case. The species isolated showed characteristic morphological features of aspergillus fumigatus but was slow sporulating. In the absence of definitive molecular sequencing methods, in this case the isolate would best be labelled as aspergillus spp. Commonly recommended treatment of aspergilloma is radical surgical decompression followed by oral voriconazole.5 Despite high doses of antifungal drugs and extensive surgery, craniocerebral aspergillosis is difficult to treat and has very high mortality rates.1,5 Intracranial fungal infection in the immunocompetent patient may fare better.1 Oral voriconazole was started in the post-operative period in this case. It is believed that neurosurgical intervention debulks the infected tissue eliminating foci of low drug penetrance.5 In a study evaluating treatment modalities of CNS aspergillosis, patients undergoing neurosurgical intervention showed better clinical outcome.5 The present case corroborates that surgical resection coupled with antifungal therapy gives a good outcome. Our case report further strengthens therapeutic management strategy in non-opportunistic aspergilloma. Since the last one year, the patient is on follow up. The patient is currently asymptomatic and completely recovered.
To conclude, this case of chronic invasive intracranial aspergilloma in an immunocompetent patient raises questions of significant clinical import. These include immunopathological mechanisms responsible for this infection, route of infection, environmental factors and genetic association. Further studies on larger cohorts are required to elucidate the exact pathogenesis.
Acknowledgments
The authors would like to express gratitude to Dr. Shweta Bhatnagar, Consultant radiologist for reviewing the radiological images pertaining to the present case.
Footnotes
Disclosure
The authors declare no conflicting interests, support or funding from any drug company.
- Received August 20, 2015.
- Accepted January 20, 2016.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.