Methanol is a highly toxic alcohol with a smell and taste similar to ethanol. Small amount around 50 - 100 ml causes permanent blindness and severe neurological dysfunction leads to death.1 More than half of methanol related morbidity and mortality is classified as accidental and therefore preventable. In addition, it can be suicidal by ingestion of a variety of commercial paint thinners, gasoline anti-freeze, windshield products, organic solvents, shellac varnish, washer fluid, photocopying fluids, perfumes, and in some eau de cologne. Occasionally, it is due to the fraudulent adulteration of wine or other alcoholic beverages.2 Its ingestion causes high anion gap metabolic acidosis from the production of formic and lactic acids and central nervous system disturbances ranging from inebriation and drowsiness to obtundation, seizure and coma. Selective toxicity of the optic nerve and basal ganglia are well-known features. Bilateral putaminal necrosis is often recognized radiologically in severe methanol toxicity and usually death occurs within 3 days.2 Here, we report a patient presented with severe high anion gap metabolic acidosis due to methanol intoxication who developed intracerebral bleed during treatment. A 47-year-old man was brought to our hospital by ambulance presented with body pain, nausea, vomiting, and blurring of vision one day ago and sudden loss of vision upon arrival to emergency department. Briefly after admission, became unresponsive, Glasgow Coma Scale 4 with dilated non reactive pupils. His blood pressure was 110/60 mm Hg, heart rate was 95/min, respiratory rate was 32/min (Kussmul’s-Kien breathing), and temperature was 36C.
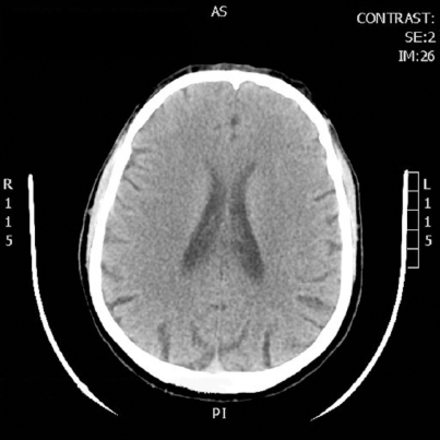
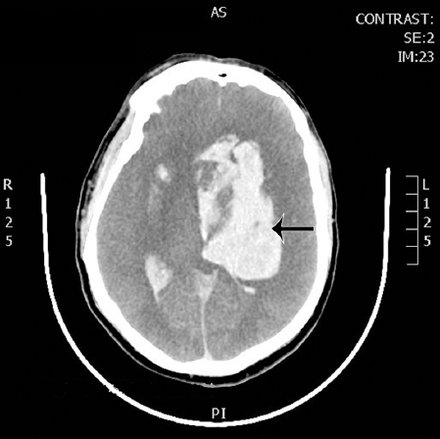
An examination of the chest, heart, and abdomen was unremarkable. He was intubated in emergency department and diagnostic work-up started. Chest x-ray was normal, computed tomography of brain showed only atrophic brain changes otherwise normal, (Figure 1), but the Blood Gases showed severe high anion gap metabolic acidosis with the following laboratory results PH 6.78 normal range=7.35-7.45, PaO2=195 normal range=75-100 mmHg, PaCO2=22 normal range=35-45 mmHg, HCO3=3.1 normal range=22-26 mQ/L, anion gap=36 normal range=12. Serum sodium=145 mmol/l normal range=135-145, potassium=5.6 mmol/l normal range=3.5-5, chloride=106 mmol/l normal range=92-112, ALT=172 normal range=37, AST=208 normal range=65, GGT=596 normal range=30, Hepatitis B surface antigen, Hepatitis C antibodies and HIV antibodies are non reactive, the elevated liver enzymes are attributed to the hypotension and hypoperfusion (ischemic hepatopathy). Ultrasound of the abdomen was performed which was unremarkable. Kidney functions, complete blood count, random blood sugar were normal. serum lactate was 7.8 nmol/L normal range=2.4 nmol/L. History from the family was taken; he is smoker, alcohol and drug abuser for almost 3 years. So history, clinical presentation, and laboratory parameters guided us to the diagnosis of methanol intoxication. Toxicology screening was carried out and the result was with methanol level 413 mg/dl normal range <10 mg/dl, salicylate and paracetamol were negative. He was started on intravenous sodium bicarbonate to correct acidosis. Ethanol was added to block formic acid production, while a hemodialysis session without heparin was initiated to help correct the severe acidosis and eliminate both methanol and its metabolites -formic acid. Follow-up arterial blood gases showed improvement of acidosis. Shortly after admission to Intensive Care Unit, he became hypotensive, fluid boluses given and vasopressor started. At the same day, he developed generalized tonic clonic convulsions. Fundus examination revealed oedematous optic disk with dilated peripapillary vessels. Second day to admission, his conscious level deteriorated with Glasgow Coma Scale 3, pupils dilated and fixed. He started to be polyuric (250-300 ml/h), central diabetes insipidus was suspected versus high fluid intake, so ethanol and sodium bicarbonate were stopped. Hemodialysis was continued for 2 hours. At noon, he started to have no gag or cough reflexes and no spontaneous breathing. Furthermore, he developed hypernatremia (serum sodium 159 mmol/l). Hemodynamics maintained on noradrenaline up to 20 micg/min. Electroencephalogram showed low voltage, no epileptiform discharge, and metabolic/hypoxic insult. computed tomography of brain was carried out and showed massive intracerebral bleed, putaminal and intraventricular, subarachnoid hemorrhage, and brain edema with midline shift (Figure 2). Third day, diagnostic criteria for brain death initiated and brain death with central diabetes insipidus was diagnosed, desmopressin with dextrose water 5% was continued. At ninth day, the patient went into cardiac arrest and pronounced dead.
Showing the first computed tomograph brain that was carried out with no abnormalities.
Computed tomography brain showing massive cerebral bleed in the basal ganglia; putamen, intraventricular bleed, subarachnoid hemorrhage, brain edema with midline shift.
In the absence of treatment, an ingestion of approximately 1gm/kg of either methanol or ethylene glycol (namely parent alcohol) is considered lethal and serious toxicity has been reported following ingestion of as little as one teaspoonful of methanol.1 The clinical features of methanol intoxication usually occurs after a latent period of 12-24 hours following methanol ingestion. The latent period corresponds to the time period in which methanol is converted by alcohol dehydrogenase in the liver to formic acid, the metabolite that is responsible for the acidosis and the toxic effects.3
Early manifestations of methanol toxicity include inebriation, nausea, vomiting, abdominal pain, headache, dizziness, and weakness. While the late manifestations (after 24 hours of ingestion) are due to the toxic effects of formic acid on brain (seizure, coma, death).3 Visual manifestations include blurred vision, light flashes, central scotomas, and blindness or optic atrophy,3 in addition to high anion gap metabolic acidosis.1 Optic nerve atrophy is due to the demyelination that results from the inhibitory effect of formic acid on mitochondrial ATP production as well as from anoxia secondary to disc hyperemia/edema.2
Methanol intoxication affecting the basal ganglia and subcortical region by ischemia and necrosis or hemorrhage especially putamins with unknown etiology thought to be related to the metabloic demands for that area. In 1988, Phang et al4 observed extensive necrosis and hemorrhagic necrosis in the basal ganglia, and hemorrhage into the ventricles of the brain in 6 of 21 patients with methanol intoxication who had a computed tomography (CT) scan of the brain. Rubinstein D et al,5 reported putaminal and subcortical white matter necrosis in Magnetic resonance imaging (MRI) of a patient with methanol toxicity in whom the initial computed tomography CT scan was normal.
The precise mechanism of necrosis and hemorrhage in methanol toxicity remains a matter of debate.2 It could be due to the direct toxic effect of methanol and its metabolite “formic acid” or secondary to anoxia and acidosis, Moreover, Phang et al4 suggested that heparin use in hemodialysis may enhance hemorrhage in necrotic areas of the brain, that is why we performed the hemodialysis without heparin.
The management of methanol toxicity may be directed towards achieving 2 goals: First, to reduce the metabolism of methanol into formic acid by inhibiting the enzyme “Alcohol dehydrogenase” using either the specific antidote “fomipizole” intravenously or ethanol intravenous/orally. Second, to remove both, parent and toxic metabolites from the blood and correct metabolic acidosis by hemodialysis and sodium bicarbonate infusion.1 Hemodialysis is usually continued until plasma levels fall below the toxic range and metabolic acidosis is corrected; hence continuous renal replacement therapy may also be considered.2 This patient suffered from a severe methanol intoxication complicated with brain hemorrhage that resulted in death of the patient.
In conclusion, cerebral hemorrhage is a rare complication of methanol intoxication with aggressive clinical course which should be considered during management of such cases.
- Received September 13, 2015.
- Accepted February 24, 2016.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.