Abstract
Objectives: To assess the efficacy and safety of rituximab for multiple sclerosis (MS) treatment in terms of reduction in clinical relapses, magnetic resonance imaging (MRI) activity, Expanded Disability Status Scale (EDSS) score and adverse events.
Methods: This is a retrospective cross-sectional study conducted at King Abdullah Medical City, from January 2017 to August 2021, involving patients with MS given rituximab, with 1-year follow-up. Clinical parameters were noted pre- and post-treatment to determine efficacy; adverse events were noted to analyze safety. A paired samples t-test was used to compare responses pre- and post-treatment. A p-value<0.05 was considered significant.
Results: Among 31 patients, 6 (19.4%) had progressive MS, and 25 (80.6%) had relapsing-remitting MS (mean disease duration=8.12±5.65 years). The annual relapse rate reduced from 1.67±0.97 to 0.06±0.25 (p<0.001), the EDSS score from 3.16±2.14 to 2.80±2.28 (p=0.141) and the MRI activity score from 1.84±1.03 to 1.03±0.18 (p<0.001). Only one patient had enhancing lesion activity post-treatment. The commonest side effect was urinary tract infection (25.8%). Only 2 patients discontinued the drug.
Conclusion: Rituximab is an efficient drug in reducing the annual relapse rate and MRI activity of patients with MS, with few tolerable side effects not leading to drug discontinuation or any lethal outcome.
Multiple sclerosis (MS) is a condition of the central nervous system carrying a chronic course and having autoimmune etiology. The disease has a prevalence of 40.4 per 100,000 people in Saudi Arabia.1 MS can have a relapsing-remitting course which begins with an acute attack and is then followed by full or partial recovery (also known as relapsing-remitting MS [RRMS]). Alternative clinical presentation of MS is characterized by progressive neurological worsening without any acute attack (also known as primary progressive MS). Secondary Progressive MS is another advanced stage in the course of disease where disability worsens gradually without a new relapse.2,3 The disease process was earlier thought to be mediated by T cells, but research has brought forth the suggestion that B cells, too, do play a role in the pathological process.4 It is now well understood that antigen presentation by B cells is a necessary step in the pathogenesis of the immune-mediated process against the glycoprotein myelin found in central nervous system.5 Therapies targeting T cells (for example, interferon-beta and natalizumab) have been traditionally used for MS, but not all patients improve despite treatment compliance. Moreover, interferon causes a myriad of highly stressful side effects, and natalizumab, in addition to causing minor adverse effects, has a risk of causing a serious condition like progressive multifocal leukoencephalopathy (PML).6 New therapies that target B cells are increasingly being investigated. Rituximab (RTX) is one such drug that targets CD 20 expressing B cells and also reduces T cells. The drug has shown promising results in the treatment process.7,8 The safety of RTX usage is established by Class IV evidence.9 Phase 2 trials have shown that RTX reduces magnetic resonance imaging (MRI) inflammatory lesions by up to 88% in the patients of MS.10,11 Off-label RTX usage in MS is further supported by several trials.11,12,13 One such study showed MRI activity reduce from 88% to 8.3% in only a year and annual relapse rate reduce from 0.75 to 0.36 with the use of RTX.13 The common side effects of RTX usage in patients with MS include infections (36%), with urinary tract infection being the most common, and infusion reactions (8%). This CD 20 targeting drug also increases the risk for fungal infections.14,15
Studies comparing the efficacy of RTX to conventional therapies have concluded that RTX has better performance in MS, especially in newly diagnosed cases of RRMS.16 Our aim was to study the reduction in MRI inflammatory lesions, disability changes and relapses in patients with MS presenting at King Abdullah Medical City as a result of RTX therapy.
Methods
We conducted a retrospective cohort study after obtaining permission from King Abdullah Medical City’s ethical review board. The study was in accordance with the principles of Helsinki Declaration. All the patients were informed about the off-label use, side effects and details of follow-up with this drug treatment. The decision to start rituximab was made by consultant panel having more than 10 years of experience in the field based on nature of the disease and their clinical experience. All patients admitted to the institution from 1st January 2017 to 31st August 2021 who received a diagnosis of MS as per McDonald’s criteria17 and were given RTX, with a follow-up of at least one year after the therapy, were enrolled in our study. Patients with MS on RTX therapy who could not be followed up were excluded from the study.
The data were collected from patients’ records and transferred to an electronic collection form that did not show any nominative information. Patients were given, in records, serial study codes and initials. These were related to the patients’ names and their registration number in a log sheet that was kept in a safe, locked place.
We identified 44 patients on RTX therapy. Thirteen of them could not be followed up and were not included in the final analysis. A sample size of 31 patients was used. Demographic details such as age and gender were noted. Clinical parameters such as duration of MS, MS phenotype, history of disease-modifying therapy (DMT) if used, indication for RTX use, clinical relapse, MRI lesions and Expanded Disability Status Scale (EDSS)18 scores at baseline and after one year were recorded. MRI lesions were defined to be either a new/enlarging lesion or Gadolinium enhancing lesion. The reporting of MRI was done by a consultant radiologist having more than 5 years of experience in the field. One gram of rituximab was given intravenously once, and then the dose was re-administered after 2 weeks. The same RTX dose was repeated after 6 months in two cycles, 2 weeks apart. The details of adverse events were also noted. Both the efficacy and safety profile of the drugs were recorded.
Efficacy was described in terms of reduction in clinical relapses, MRI activity and EDSS at one-year follow-up after RTX therapy when compared to the baseline before the start of treatment. The safety profile of RTX was defined by the adverse events during treatment.
The primary end point was the efficacy of RTX, whereas the safety of the drug was also a target to be assessed. All collected data were analyzed using SPSS version 25.0. All categorical variables (such as gender, MS phenotype, history of DMT and occurrence of clinical relapse) were calculated as percentages and frequencies, whereas quantitative variables (age, duration of diseaseetc) were recorded as mean and standard deviation. P-values were found to depict the efficacy of the drug by applying a paired samples t-test after comparing the number of clinical relapses, MRI activity and EDSS before and after the treatment at one-year follow-up. A p-value<0.05 was labeled significant.
Efficacy was described in terms of reduction in clinical relapses, MRI activity and EDSS at one-year follow-up after RTX therapy when compared to the baseline before the start of treatment. The safety profile of RTX was defined by the adverse events during treatment. The primary end point was the efficacy of RTX, whereas the safety of the drug was also a target to be assessed.
All collected data were analyzed using SPSS version 25.0. All categorical variables (such as gender, MS phenotype, history of DMT and occurrence of clinical relapse) were calculated as percentages and frequencies, whereas quantitative variables (age, duration of diseaseetc) were recorded as mean and standard deviation. P-values were found to depict the efficacy of the drug by applying a paired samples t-test after comparing the number of clinical relapses, MRI activity and EDSS before and after the treatment at one-year follow-up. A p-value<0.05 was labeled significant.
Results
Clinical and demographic features
Of the 31 patients who completed the treatment and follow-up and were included in the final analysis, 12 (38.7%) were male and 19 (61.3%) were female. The average age of the patients was 36.80±8.04 years, with a minimum of 23 years and a maximum of 54 years. There were 6 (19.4%) patients with progressive MS including both primary and secondary progressive MS, and 25 (80.6%) had RRMS. The mean disease duration was 8.12±5.65 years. Nine (29%) patients were treatment naive, and 22 (71%) were previously treated with DMT. Eight (25.8%) patients were being treated with fingolimod, 7 (22.6%) with interferon, 5 (16.1%) with teriflunomide, 1 (3.2%) with dimethyl fumarate and another 1 (3.2%) with natalizumab. Various reasons to start RTX were clinical relapses (12.9%), MRI activity (25.8%) or both (32.3%) instead of treatment of aggressive disease (9.7%), side effects of previous drugs (12.9%) and the personal preference (6.5%) of the patient. The distribution of these variables is given in Table 1.
- Distribution of clinical and demographic variables.
Efficacy. Clinical relapse
Before treatment, relapse was present in 26 (83.9%) patients, with an average of 1.67±0.97 relapses per year, whereas 5 (16.1%) patients did not have any clinical relapse even before treatment. The post-treatment mean relapse was 0.06±0.25 relapses per annum. Only 2 (6.5%) patients had clinical relapse after 2nd and 3rd cycle of treatment. There was a significant difference in relapses both before and after the treatment (p<0.001).
MRI Activity
Baseline MRI activity was stable in 16 (51.6%) patients before treatment. Out of 15 (49.4%) patients showing MRI activity, 7 (22.6%) had new lesions, and 5 (16.1%) had enhancing lesions on MRI. There were only 3 (9.7%) patients who had both new and enhancing lesions. The average score of MRI activity was 1.84±1.03, reducing to 1.03±0.18 after the treatment. All the patients had stable MRI activity, except one (3.2%), at one-year follow-up after RTX treatment. There was a significant reduction in MRI activity (49.5% vs. 3.2%) after treatment (p<0.001).
EDSS
The mean EDSS scores were 3.16±2.14 and 2.80±2.28 before and after the treatment. There was a reduction in EDSS score, but it was not statistically significant (p=0.141). A tabulated display of the efficacy of RTX is shown in Table 2.
- Efficacy data of rituximab after one-year follow-up
Safety
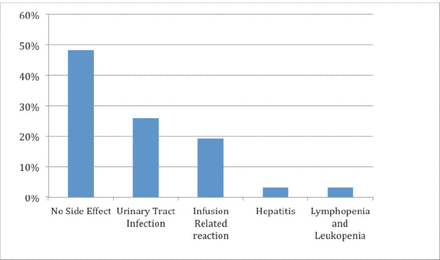
Fifteen(48.4%) patients did not have any side effects, with good tolerability. Eight (25.8%) patients developed urinary tract infection, which was the most commonly observed side effect. Six (19.3%) patients had infusion-related allergic reactions, and among them, only one had a severe reaction in the form of serum sickness that led to discontinuation of the drug. The remaining 5 had mild infusion-related reactions (4 had throat irritation and pruritis and one had hypotension) that resolved after stopping the infusion and then continuing it at a slower rate. One patient had hepatitis and discontinued the medication, and another patient had leukopenia and lymphopenia. Only 2 (6.5%) patients discontinued the therapy due to side effects of the drug.
The detailed frequencies of side effects of RTX therapy are shown in Figure 1.
- Graph showing the details of adverse events of rituximab.
Discussion
Recent evidence has suggested that RTX is a highly effective and, in many ways, superior form of DMT for patients suffering from MS.16 The effect of rituximab is mediated by its activity on both B and, to a lesser extent, T lymphocytes. The pathways include immunosuppression by decreasing the antigen presentation by B cells and a reduced production of autoantibodies.19
Our study corresponds to recent RTX findings indicating that the drug significantly reduces MRI activity of the disease and the annual relapse rate. Our results were seen in a sample comprising patients with progressive MS (19.4%) and RRMS (80.6%). A remarkable drop in annual relapse rate from 0.84 to 0.06 was observed (p<0.001). Our findings are similar to those of Bellinvia et al.13 In their observational study published in 2020 that showed the annual relapse rate reducing from 75% to 36% after treatment. In 2008, Hauser et al9 conducted phase II clinical trials that produced the same conclusion regarding a reduction in the annual relapse rate of MS. It has been demonstrated in previous studies that RTX reduces the pathological activity of the disease seen on MRI.7 Our study resonated with the previous studies, as new or enhancing lesions on MRI were significantly reduced from 49.5% to 3.2% after the one-year follow-up. We considered the frequency of new or enhancing lesions on MRI as a marker of disease severity. The same marker was used by Bellinvia et al13 in their observational study.
The EDSS is used to monitor the disability seen in patients diagnosed with MS in a quantitative manner. Our research showed no significant changes in EDSS scores. The mean EDSS score was found to have reduced from 3.16 to 2.81in one year (p=0.141). The almost unchanged EDSS score found in our study is in line with previous research in the area.20 There have been conflicting reports on whether EDSS score ameliorates, remains stable or even worsens after RTX therapy.21
Combined with the data and results of previous studies and trials, our research findings indicate that RTX has good efficacy in the treatment of MS, considering MRI disease lesions and the annual relapse rate as markers for disease activity. However, the effect of RTX in reducing EDSS is not that obvious, although our study follow-up of these patients might reveal significant improvement in EDSS. However, this hypothesis needs to be evaluated in future research.
The evaluation of the safety profile of RTX in our sample population revealed promising results. In majority of the patients (48.4%), no adverse event was observed. The most common side effect observed was urinary tract infection, which was seen in 25.8% of the patients treated with RTX. Only 6 patients (19.4%) experienced infusion-related adverse reactions. This is in contrast to previous studies and trials that have reported infusion-related reactions to be the most common side effect of RTX usage.22 Other less frequent side effects included hepatitis, lymphopenia and leukopenia; only 2 patients (6.5%) had to discontinue the treatment due to these side effects. Immunosuppression, as a result of B-cell-depleting therapy, is the obvious cause of the increased incidence of infections among patients treated with RTX. For this reason, it should be common practice to evaluate all patients who develop infection secondary to RTX therapy for leukopenia. An alteration in the management might be employed by prolonging the dosing intervals to reduce susceptibility to infections. Another treatment approach for patients who develop reduced plasma IgG antibody levels is replacement therapy in the form of immunoglobulin. This can also have a favorable effect in reducing the incidence of infections. None of our sample patients developed an opportunistic infection secondary to the therapy.
No case of PML was reported. It is interesting to note that previous studies have reported that RTX therapy leads to PML. Other associated risk factors for the development of this condition, include rheumatoid arthritis, lymphoma and chemotherapeutic agents.23,24 Combined with the findings of previous trials, it can be concluded that RTX is at the very least as safe as any other disease-modifying drug used for the treatment of MS. B-cell depletion has a known side effect of reducing vaccine efficacy.25 Therefore, we put forward the recommendation of updating one’s vaccination schedule and getting vaccinated against pneumococcal infections before the start of RTX therapy.
Twenty-five (80.6%) of our enrolled cases were diagnosed with RRMS. The most common indications for starting RTX therapy in our study population were MRI activity (25.8%), clinical relapse (12.9%), and a combination of the two (32.3%). This has been reported in previous studies as well.7,13 Other less common indications included side effects of previous medication (12.9%), aggressive disease (9.7%) and patient preference (6.5%).
Most of the patients (29%) we studied were not taking any previous disease-modifying drug for MS. The most common DMTs used by the patients included fingolimod (25.8%), interferon (22.6%) and teriflunomide (16.1%). Other drugs were diethyl fumarate and natalizumab (each 3.2%). It should be noted that we studied the effects of RTX as monotherapy similar to most of previous studies. However, some studies have evaluated the effect of RTX as add-on therapy, and they reached similar conclusions: RTX is an effective therapeutic option as add-on therapy for patients with MS.10
Limitations
Being an observational study, this research had the inherent limitation of not accommodating a control group for comparison. The data was limited and insufficient in reaching a sweeping conclusion. As the study was based at only one center, there was an inherent selection bias. Nevertheless, our results and conclusions are comparable to those of previous studies, as highlighted in the Discussion section. A randomized controlled trial with a large population is recommended.
Conclusions
Rituximab is an effective and safe option in reducing the annual relapse rate and the disease activity observed on MRI in patients having the diagnosis of MS, with good tolerance and few side effects. However, RTX has not been shown to significantly reduce EDSS score.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received October 13, 2021.
- Accepted March 15, 2022.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.