Abstract
Objectives: To evaluate all the coincidence cases of Guillain-Barré syndrome (GBS) and myasthenia gravis (MG).
Methods: We performed web-based research of the overlapping incidence of GBS and MG in studies occurring from 1982 to 2016 and restricted to the English language.
Results: Among 15 cases, an elevated CSF protein level without pleocytosis was found in 10 cases (66.7%); reduced nerve conduction was found in 13 cases (86.6%); a positive repetitive nerve stimulation test occurred in 11 cases (73.3%); anti-AChR antibodies were found in 13 cases (86.6%); anti-GQ1b antibodies were found in 6 cases (40%); a positive edrophonium chloride test was present in 10 cases (66.7%); and a co-occurring thymoma or thymectomy occurred in 4 cases (26.6%). The MG co-occurred with acute inflammatory demyelinating polyneuropathy (AIDP) in 8 cases and with Miller Fisher Syndrome in 5 cases. Treatment in the assessed cases included pyridostigmine (10 cases), prednisolone (7 cases), intravenous immunoglobulin (9 cases), plasmapheresis (3 cases), combined intravenous immunoglobulin and plasmapheresis in one case, and immunosuppressive drugs in 2 cases (azathioprine). Functional outcome was mentioned in 13 patients. The prognosis was favorable in 8 of the 15 recorded patients (Hughes 0-1), and 2 cases resulted in death.
Conclusion: Although comorbidity of GBS and MG is extremely rare, early recognition of this combination of inflammation of peripheral nerves and the neuromuscular junction is of great importance for both initial treatment and a better prognosis.
Myasthenia gravis (MG) is an autoimmune disorder caused by antibodies against acetylcholine receptors (AchR) targeting the neuromuscular junction, resulting in muscle weakness and fluctuating fatiguability.1 It has distinct immunogenic characteristics and can also be considered a paraneoplastic syndrome associated with thymoma or thymic hyperplasia. Current available treatments include symptomatic pharmacological treatment, immunomodulatory drugs, plasma exchange, thymectomy and other supportive therapies. The prognosis is relatively favorable, with less than five percent mortality.
Guillain-Barré syndrome (GBS) is the most common and most severe acute paralytic neuropathy and is mediated by autoantibodies against myelin proteins or axonal components of peripheral nerves. If unrecognized or overlooked, GBS is associated with high rates of mortality due to acute progressive weakness or respiratory failure. The most common symptoms include limb weakness, areflexia and paralysis. There are several recognizable variants of GBS, including acute inflammatory demyelinating polyradiculoneuropathy (AIDP), Miller Fisher Syndrome (MFS), acute motor axonal neuropathy (AMAN), and acute motor-sensory axonal neuropathy (AMSAN). One of the most common subtype of GBS, MFS is an immune-mediated neuropathy that involves the triad symptoms of acute ophthalmoplegia, ataxia and areflexia in the presence of anti-GQ1b antibodies. Several treatments exist, such as plasma exchange and the administration of intravenous immunoglobulin (IVIG).2
As is well-known, MG and GBS are different autoimmune disorders, affecting the neuromuscular junction and peripheral nerve, respectively. However, the exact pathophysiological process of both MG and GBS remains unclear. It is estimated that the frequency of co-occurrence of MG and GBS is less than 1 in 10 billion.3 To the best of our knowledge, the occurrence of MG and GBS overlap syndrome is quite rare.3-14 Furthermore, only 4 studies have reported the comorbidity of MG and MFS previously.15-18 Here, we review all previously described cases and present a new case of our own. We also aim to summarize the clinical features and to elucidate the cause underlying such a rare overlap syndrome.
Methods
Literature was reviewed using PubMed, Embase, the Cochrane Library and Science Direct from January 1982 to December 2016, and the articles were restricted to those published in English. Key search terms included “Guillain-Barré syndrome”, “acute inflammatory demyelinating polyradiculoneuropathy”, “miller fisher syndrome”, “acute motor axonal neuropathy”, “acute motor-sensory axonal neuropathy” and “myasthenia gravis”. Patients with combined GBS and MG were identified and their clinical data (such as gender, age, nationality, past history, precipitating factors, clinical presentations, laboratory examinations, CSF findings, variants of GBS, anti-AChR antibody presence, anti-GQ1b antibody presence, thymoma, treatment and outcome) were all comprehensively evaluated. Descriptive statistics were utilized to determine the characteristics of these entities, and their respective frequencies were expressed as percentages.
Results
Of the 15 patients in the cases assessed, 6 were female and nine were male. All patients were aged 17-90 years. There were seven Chinese patients, 3 Israeli, 2 American, one Japanese, one Caucasian and one French. Of the 15 patients, 10 had precipitating factors such as upper respiratory infection, fever or watery diarrhea. Most cases had similar symptoms, including extraocular muscle weakness, ptosis and areflexia. An elevated CSF protein level without pleocytosis was found in 10 cases (66.7%); reduced nerve conduction was found in 13 cases (86.6%); a positive repetitive nerve stimulation test occurred in 11 cases (73.3%); anti-AChR antibodies were identified in 13 cases (86.6%); anti-GQ1b antibodies were found in 6 cases (40%); an edrophonium chloride test was positive in 10 cases (66.7%); and a co-occurrence with thymoma or previous thymectomy was present in 4 cases (26.6%). According to the variants of GBS, overlap of MG with AIDP occurred in 8 cases, overlap of MG with MFS in 5 cases, and overlap of MG with AMAN and AMSAN each once. The involved treatments included pyridostigmine (10 cases), prednisolone (7 cases), IVIG (9 cases), plasmapheresis or plasma exchange in 3 cases, combined intravenous immunoglobulin (IVIG) and plasmapheresis in one case, and immunosuppressive drugs in 2 cases (azathioprine). Functional outcome was mentioned in 13 patients and was ranked according to the adopted scale by Hughes. The prognosis was relatively favorable in 8 of the 15 recorded patients (Hughes 0-1). Two cases resulted in death (one had a diagnosis of AIDP and the other had a diagnosis of AMAN).5,9
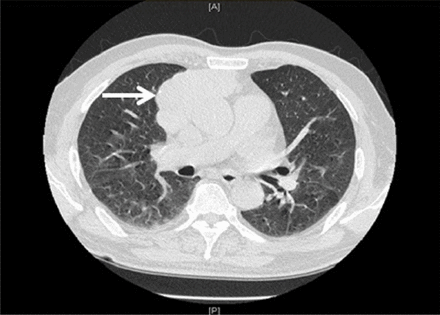
High-resolution chest CT revealed a large 8 cm×3.3 cm thymoma.
The demographic data and characteristics of comorbid AIDP and MG (8 cases).
The demographic data and characteristics of comorbid MFS and MG (5 cases).
Discussion
The GBS and MG are well described heterogeneous autoimmune disorders characterized by the presence of autoantibodies against several different antigens in peripheral nerves and neuromuscular junctions. The incidence of MG is 10-20 cases per million persons per year and that of GBS is 0.4-1.7 cases per million persons per year. Thus, the co-occurrence of both diseases is extremely rare. Although GBS and MG may have some clinically similar symptoms and neurophysiological findings, the differential diagnosis should be made on the basis of ptosis with or without ophthalmoplegia, distribution of limb weakness, and reflexes. The typical clinical characteristics of GBS and MG may be helpful in diagnosis of this type of overlap syndrome.
In our case study, MG was diagnosed according to clinical features, electrophysiological data, a positive neostigmine test, the presence of anti-AChR antibodies and radiological findings of thymoma. The diagnosis of MFS was established on the basis of the acute clinical course, nerve conduction studies indicating demyelinating polyneuropathy, albuminocytologic dissociation in the CSF, the presence of the crucial triad (ophthalmoplegia, areflexia, ataxia), and the positive anti-GQ1b antibodies. As a result, according to the clinical characterization, electrophysiological results, laboratory data, the improvement of symptoms with anti-acetylcholinesterase and IVIG treatment, and especially, improvement of symptoms upon thymectomy, the diagnosis of both MG and MFS was established.
The demographic data and characteristics of AMSAN (1 case) and AMAN (1 case).
Fifteen patients, 9 males and 6 females, were observed. Most of the patients presented with precipitating factors. In accessory examinations, the clinical features, from most to least common, were the presence of anti-AchR antibodies (86.6%), a positive nerve conduction test (86.6%), a positive repetitive nerve stimulation test (73.3%), an elevated CSF protein level in the absence of pleocytosis (66.7%), a positive edrophonium chloride test (66.7%), and the presence of anti-GQ1b antibodies (40%). Furthermore, 4 different variants of GBS were observed, 8 cases of AIDP, 5 of MFS, one of AMSAN and one of AMAN. Immunotherapy treatments included IVIG or plasma exchange, and most of the patients had a good prognosis.
Autoimmunity may play a vital role in the pathology underlying both MG and GBS. First, molecular mimicry, which suggests similarity between infectious agents and self-antigens may initiate concurrent MG and GBS, has been suggested as a possible hypothesis.3 It was proposed that some antibodies may show cross-reactions against both myelin proteins on peripheral nerves and acetylcholine receptors in neuromuscular junctions.19 Some clinical evidences also support the idea of molecular mimicry between gangliosides (such as GQ1b for MFS) and antecedent infectious agents in patients with GBS. Such a theory has been supported by an experimental study showing that antibodies against AChR from the serum of GBS patients cross-reacted in mice.19 Second, it has been reported that approximately 8-15% of all MG cases are complicated by autoimmune diseases, such as immune thyroid disease and collagen disease.20 The association of MG or GBS with autoimmune diseases, such as autoimmune thyroiditis, has been previously described.5,8,12
Another hypothesis proposed is that thymoma or thymus hyperplasia-associated multi-organ autoimmunity may also play an important role in the process of autoimmunity. Considering the fact that 4 patients suffered from thymoma in the cases we reviewed, thymoma may be considered to be a condition commonly involved in MG. Lastly, some precipitating illnesses are thought to be driving factors in initiation of autoimmune disorders. If an infection occurs, it may not only induce antibody production to initiate GBS but may also enhance the production of anti-AChR antibodies in neuromuscular junctions, leading to MG.21
In summary, the coincidence of GBS and MG should be considered when the presenting features do not fully fit one disease or the other. Although some possible hypotheses have been raised, the underlying mechanisms may warrant future investigation.
Footnotes
Disclosure. This study was funded by the National Natural Science Foundation of China (81271309, 81301016, 81541129) and the Beijing Municipal Administration of Hospitals’ Youth Programme (QML20150303).
- Received April 16, 2017.
- Accepted October 25, 2017.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.