Coronavirus disease 2019 (COVID-19) emerged in late 2019 in Wuhan, China.1 The outbreak was confirmed as a pandemic on February 11, 2020, by the World Health Organization (WHO). By the end of April 2020, more than 3.5 million people were infected worldwide. The number of affected people in Saudi Arabia has reached more than 28,000 at the time of this report’s submission. Since the early phase of the novel coronavirus epidemic, the Ministry of Health (MOH) in Saudi Arabia has been working to develop strict measures to contain the virus spread, going as far as locking down certain cities and restricting movement within and between others. The pandemic has affected all medical specialists’ ability to practice efficiently and has resulted in a major change in the clinical pathways of managing people with epilepsy worldwide.1 The Saudi Epilepsy Society (SES), in collaboration with the Saudi Patient Safety Center, has initiated a priority care classification in epilepsy management practice. In addition, the SES COVID-19 task force has established a consensus for the best evidence-based practice related to epilepsy during the pandemic.
Epilepsy care priorities during the COVID-19 pandemic
Medical care delivered to patients with epilepsy in Saudi Arabia is usually carried out in various centers across the country. During the current pandemic, access to many of these centers might be altered and restricted. As a result, patients can receive medical care through different channels such as local medical facilities, virtual clinics, and remote delivery of antiepileptic medications. Nonetheless, certain conditions may still necessitate that patients visit tertiary care centers and/or emergency rooms.
To provide guidance to the health-care systems, health-care providers, and patients with epilepsy, the SES has defined clinical priority classes that will each receive care in the proper medical setting. The potential epilepsy presentations were categorized as acute, intermediate, and chronic and were assigned respective clinical care priorities (A, B, C, & D), as shown in Table 1. Priority A includes life-threatening presentations that must be seen in an emergency room immediately. These presentations include status epilepticus, acute repetitive seizures, and seizure clusters. Priority B includes presentations that are relatively urgent but not immediately life-threatening. This category includes single, first-time, and breakthrough seizures that do not meet the criteria for status epilepticus. These patients deserve an evaluation within seven days in the outpatient setting. Priorities C and D represent patients who do not require an urgent evaluation, including patients who have febrile seizures and relatively controlled epilepsy. These classes serve as a general guideline for practitioners but should not replace the clinical judgment of the physician evaluating a given patient during this time.
Prioritization of medical care based on clinical category during COVID-19 pandemic.
Clinical follow-Up visits and telemedicine
For priorities B, C, and D, care is likely to be required on an outpatient basis. Although epilepsy clinics across the country have restricted their services, ensuring continuity of care is critical during this difficult time to prevent destabilization of patients. It is recommended that regular physical patient visits be replaced by virtual telemedicine clinics as much as possible. Telemedicine visits may occur in the form of a private audiovisual teleconference call or a direct phone call, which may suffice in some situations. It is important to document the visits accurately to ensure care continuity and to ensure the legitimacy and legality of the visit.
Access to antiepileptic medications
In the context of a national curfew and restrictions on people’s movement, maintaining access to antiepileptic medications is vital. Medical supplies can be maintained through channels such as medication renewal and remote delivery by carriers or through the mail service. If certain medications and/or remote prescribing and delivery are not available at certain centers, shifting resources between health-care centers, where possible, may resolve these problems. An alternate solution to the problem of unavailability of a certain antiepileptic is that the treating epileptologist could consider switching to a medication that is readily available and fulfills the clinical need. However, this approach needs to be considered carefully by a specialist in epilepsy because it is generally not advisable to alter a treatment regimen during the COVID-19 pandemic to avoid precipitating a clinical worsening of epilepsy.
COVID-19 and seizures
The current evidence suggests that COVID-19 only rarely increases the risk of acute symptomatic seizures.2 A recent multicentric study in the province of Hubei in China, the epicenter of the pandemic, showed no increase in risk for new seizure development, and this has also been observed in many other countries. It is well known that symptoms such as high fever can increase the risk of seizure in any person, especially in patients with epilepsy. Despite a certain group of patients with severe COVID-19 developing a neurological manifestation such as encephalitis, there is no apparent increase in the risk for seizure.2 To date, a single case was reported in Italy that lists breakthrough focal status epilepticus as the primary presenting feature of COVID-19 in a patient who has controlled epilepsy.3 In the context of the highly dynamic and rapidly changing information at the current time, clinicians are encouraged to follow the literature closely while evaluating their patients.
Epilepsy and the risk of COVID-19
There is no evidence that patients with epilepsy are at any particular increased risk for COVID-19 or that epilepsy is associated with a risk of having a severe infection. Antiepileptic medications themselves are not immunosuppressive, but the rare patients who develop a neutropenia related to their antiepileptic medications may theoretically be at increased risk. In extremely rare cases, some epileptic patients who take an immunomodulatory medication such as a steroid or another immunosuppressant may have more severe symptoms of COVID-19.
Antiepileptic medication interactions with drugs used for COVID-19
Several drugs are used in COVID-19 management, ranging from antiviral to antimalarial drugs. Many interactions are evident between antiepileptic medications and drugs used for COVID-19. In the current climate of frequently changing information, it is recommended to review the COVID-19 drug interaction literature and resources.4 Based on the aforementioned, potentially significant interactions with several drugs used for COVID-19 can occur when used with phenobarbitone, primidone, phenytoin, and carbamazepine. Expectedly, the least frequent interactions have been found with levetiracetam, lacosamide, gabapentin, retigabine, topiramate, vigabatrin, zonisamide, valproate, and lamotrigine. It is not recommended to alter the antiepileptic regimen as a potential preventive measure during the pandemic in anticipation of starting a COVID-19 medication. Consultation with an epileptologist may help upon prescribing a COVID-19 medication for a patient with epilepsy.
Epilepsy monitoring and the Epilepsy Monitoring Unit
Although the ultimate judgement to proceed with epilepsy monitoring is up to the treating physician and the health-care institution, it is recommended that elective epilepsy monitoring be rescheduled until after reasonable resolution of the pandemic. The rationale behind this recommendation is reducing exposure and aiding institutions in optimal utilization of their bed capacity. In the case of urgent outpatient electroencephalography (EEG), infection control precautions and local institutional precautionary measures are recommended. In general, the benefit of EEG must be weighed against the risk of infection.
Electrophysiology monitoring of COVID-19 patients
In the situation of EEG or any electrophysiology monitoring of a COVID-19 patient, the following measures are recommended: 1) Personal protective equipment, including at least an N95 face mask, gown, gloves, and eye protection, must be worn by the individual performing the procedure. 2) One EEG and electrophysiology machine is used exclusively for COVID-19 patients. 3) The machine should be covered with a transparent protective sheet. 4) Hyperventilation techniques should be avoided in most cases. If feasible, centers should consider discarding electrodes and cables used for COVID-19 patients if they are not otherwise disinfectable.
Epilepsy Surgery during the COVID-19 Pandemic
Surgical procedures for epilepsy should be cancelled and rescheduled for the future. The following procedures could be considered urgent: 1) Replacement of a depleted internal pulse generator for a vagus nerve stimulator and deep brain stimulator to prevent worsening of epilepsy control. 2) Surgical treatment of infected wound or hardware. 3) Surgical procedure for a hardware malfunction. 4) In very rare cases, surgical treatment of super-refractory status epilepticus based on the clinical judgment of the treating clinicians. Standard precautions for COVID-19 should be carried out prior to admission, during admission, and during surgery. All patients must be tested for COVID-19 prior to surgery.
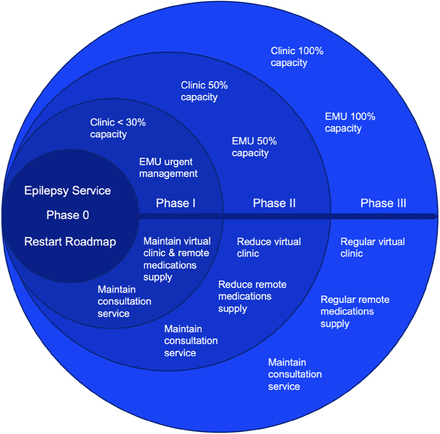
Roadmap for resumption of full epilepsy service during and after COVID-19 pandemic
The COVID-19 situation remains uncertain, and there are no definite projections as to the end of the pandemic. Some projections indicate the pandemic may go on for many months.5 Therefore, it is recommended that a roadmap based on a flexible strategy to restart epilepsy services be adopted according to the unfolding situation and the directives of the MOH and other related government authorities. The following strategies are suggested for restarting epilepsy services gradually in different phases, as shown in Figure 1. Each phase is projected to take about a month, although this rough estimate may turn out to be longer or shorter depending on the actual unfolding of events and real-time directives.
Regular epilepsy service restart roadmap during and after COVID-19 pandemic.
Phase 0
During the complete curfew phase, it is recommended that epilepsy services provide in-hospital consultations for emergencies and urgent cases only while conducting epilepsy clinics virtually by telemedicine. Epilepsy Monitoring Unit (EMU) admissions and elective surgeries are to be rescheduled with the aforementioned exceptions.
Phase I
Epilepsy clinics
When appropriate, epilepsy services may decide to initiate regular physical visits for urgent and semi-urgent cases only. The urgency is to be assessed by the treating epileptologist, taking into account any changes in patient condition. Social distancing guidelines must be followed while scheduling patients and determining clinic capacity. The clinic’s capacity should not exceed 30 percent of the regular capacity prior to COVID-19, although this will vary from clinic to clinic, depending on the pattern of practice and available resources. It is possible to postpone the scheduled check of the internal pulse generator (IPG) capacity for some patients on neurostimulator devices (such as vagus nerve stimulator and deep brain stimulator). The IPG longevity can be estimated based on the parameters obtained during the last patient visit and the duration for which the patient has had the implant. Those who require a visit are prioritized during this phase.
Virtual clinics
It is recommended to maintain and optimize virtual clinics for regular and non-urgent cases.
Medication supply
Ensure optimal antiepileptic medications are supplied. This can be assessed through a virtual clinic follow up or phone calls.
EMU
EMU services are provided only where urgent medical or surgical intervention is indicated.
Consultation services
Maintain in-hospital epilepsy service for emergency and urgent in-hospital consultations.
Phase II
Epilepsy Clinics: At this point, institutions may be able to increase the clinic capacity to 50 percent (although this percentage may vary depending upon the situation of the institution, as stated previously). Patients are scheduled in the clinics according to the clinical judgement of their priority level.
Virtual clinics
Maintain virtual clinics for any patient who has controlled epilepsy.
Medication supply
At this phase, the remote medication supply will be reduced but maintained to all patients with controlled epilepsy.
EMU:
Increase the capacity to 50 percent, based on priority.
Consultation services
Maintain in-hospital epilepsy service for emergency and urgent hospital consultations.
Phase III
Establish regular pre-pandemic epilepsy services.
Note: Movement from one phase to another can be reversed based on pandemic condition, hospital capacity, and MOH directives.
In conclusion, the COVID-19 pandemic has transformed the manner in which epilepsy services may be provided in tremendous ways by altering clinical pathways, resource utilization, and practice patterns worldwide. In this report, the practical recommendations described are based on the best available literature, evidence, and expert consensus developed by the SES COVID-19 taskforce. The COVID-19 presents a dynamically evolving situation with rapidly evolving data. These recommendations apply to the situation current to their publication, and their validity may change in the future, requiring an update. Clinicians must consult the literature regularly and use clinical judgement while applying these recommendations.
Case Reports
Case reports will only be considered for unusual topics that add something new to the literature. All Case Reports should include at least one figure. Written informed consent for publication must accompany any photograph in which the subject can be identified. Figures should be submitted with a 300 dpi resolution when submitting electronically. The abstract should be unstructured, and the introductory section should always include the objective and reason why the author is presenting this particular case. References should be up to date, preferably not exceeding 15.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.