Coronavirus disease 2019 (COVID-19) is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). The World Health Organization declared COVID-19 to be a global health emergency on January 30, 2020, and its rapid spread was declared a pandemic on March 11, 2020.1 The symptoms and severity of COVID-19 are variable, ranging from mild symptoms, including fever and cough, to more severe disease conditions, including pneumonia and acute respiratory distress syndrome, which may eventually lead to death.2 Asymptomatic carriers, who were observed in previous screenings of the disease, act as a silent source of infection to the surrounding population, and efforts should be made to identify them.3
Information related to the COVID-19 pandemic is rapidly evolving. The rapid spread of this disease is causing a significant burden on healthcare systems worldwide.4 The Saudi Ministry of Health has taken early measures to ensure the readiness of healthcare facilities in terms of bed capacity, medical staff, and supply of equipment.5 Medical institutes and health authorities, including the Saudi Center for Disease Prevention and Control (Saudi CDC), have released general recommendations and guidelines to reduce the spread of the disease.6 However, this unprecedented global situation has specifically impacted elective services and planned procedures in all healthcare facilities.7
Since the beginning of the pandemic, neuromuscular complications have also been observed. These range from headaches, myalgia, and fatigue, to seizures, myopathy, strokes, Guillain-Barre syndrome, and encephalopathy. It is difficult for physicians to determine whether neurological manifestations are directly or indirectly related to COVID-19. Patients with known neuromuscular disorders, such as spinal muscular atrophy (SMA), are prone to either the worsening of their previous neurological symptoms or the development of new neurological presentations due to COVID-19 infection.8 The Centers for Disease Control and Prevention (CDC) defines those at high risk of severe illness as the elderly population and patients with serious clinical conditions. However, there is still limited data regarding which underlying medical conditions put patients at higher risk for infection with COVID-19.9
Spinal muscular atrophy is an autosomal recessive genetic disease that causes weakness and atrophy of the affected skeletal muscles. This neuromuscular disorder is the result of the insufficient expression of the survival motor neuron protein, which causes degeneration in the motor neurons of the brain stem and spinal cord. Spinal muscular atrophy affects skeletal muscle groups, including the trunk, limbs, and eventually respiratory and bulbar muscles.10
Data is still lacking as to how patients with SMA react to COVID-19 infection. However, SMA is considered to be a disorder that may increase the risk of developing severe COVID-19 symptoms,7 especially in patients with advanced stages of SMA and respiratory muscle affection. These patients are more susceptible to infections, including COVID-19.8 The transmission of this viral infection between infected individuals and SMA patients, and vice versa, could occur through direct inhalation of infected droplets or by handling contaminated services or surfaces.11 In addition, other factors may increase the risk infection in neuromuscular patients, such as the degree of disability, weak bulbar muscles, impaired cardiac functions, kyphoscoliosis, nutritional status, administration of immunosuppressive therapies, and the existence of other comorbidities.12
Although COVID-19 appears to affect primarily adults, children of all ages can be infected.13 Previous literature recorded that 1–5% of diagnosed patients were below the age of 18.14 Moreover, the CDC estimates that up to 20% of infected children may require hospitalization and up to 2% may eventually need intensive care units.6 Since there are currently no proven therapies for the virus and no available vaccinations to prevent the infection,1 the main objective is to prevent the spread of the disease in general, and in patients with SMA specifically.7 This consensus of recommendations addresses the care of SMA patients during the COVID-19 pandemic and aims to homogenize the management strategies related to SMA patients, caregivers, and healthcare providers in the KSA.
An expert panel of 8 pediatric, adult and pediatric neuromuscular and pediatric neurology consultants across the KSA was assembled to provide unified recommendations for healthcare providers dealing with SMA patients and caregivers during this evolving public health pandemic. The panel members searched for literature related to healthcare professionals dealing with SMA patients, reviewing studies on SMA patients’ risk of contracting COVID-19; the magnitude of COVID-19 symptoms in SMA patients; and the management of SMA patients, including the administration and interruption of SMA medications, during the pandemic. They reviewed the published data using Medline, PubMed, and other clinical search engines. An initial draft for consensus recommendations was developed by the elected members of the panel and compiled statements were presented in a virtual meeting on May 7, 2020. A final document was presented in this meeting, and feedback was provided by the entire panel. Only statements that achieved consensus by the panel were included in this document (Table 1).
Consensus Statements for the Management of Spinal Muscular Atrophy in Saudi Arabia.
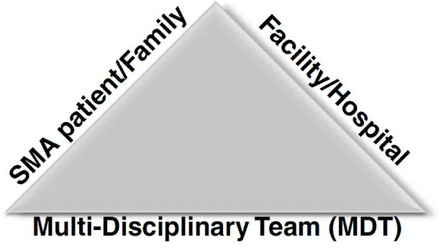
The panel members compiled all recommendations under the umbrellas of 3 main stakeholders related to SMA: (1) SMA patients and supporting families and caregivers, (2) the facilities or hospitals dealing with SMA patients, and (3) the multidisciplinary teams (MDTs) involved in the management of SMA patients (Figure 1).
Stakeholders related to the management of spinal muscular atrophy (SMA) in the context of COVID-19.
Patients with SMA and their supporting families and caregivers
The risk of COVID-19 infection in patients with SMA. There is no existing data on how COVID-19 might affect SMA patients since there are no reported cases of individuals with SMA catching COVID-19. However, patients with advanced stages of SMA could be more susceptible to infection due to weakness in their respiratory and bulbar muscles.8 Our panel of experts stated that physicians should not consider SMA patients with no respiratory weakness, bulbar dysfunction, or other risk factors to be at high risk of COVID-19 infection.8,12 However, patients and their families must exercise extreme caution to minimize the risk of infection by implementing all national and international recommendations on COVID-19 prevention.
All SMA patients infected with COVID-19 must be closely monitored to avoid a possible rapid decline in their respiratory function.8 Patients with weak respiratory muscles or those who require noninvasive ventilatory support might require a longer time to recover, and their prognosis might be more guarded.15 In addition to respiratory status, other factors that may increase the risk of SMA patients contracting severe infections include life expectancy, degree of disability, weak bulbar muscle, kyphoscoliosis, nutritional status, immunosuppressive therapies, and the existence of other comorbidities.12
Management of spinal muscular atrophy patients during the COVID-19 pandemic. The COVID-19 pandemic has reformed global health systems, including the Saudi system.16 Most outpatient visits have been canceled, elective and non-urgent procedures have been postponed, and in-hospital admissions have been restricted to life-threateningly or considerably ill patients.17 Furthermore, the uncertainty regarding the future and duration of this pandemic presents a huge challenge to the medical community.18 Thus, healthcare providers and MDTs dealing with SMA patients need to adapt and tailor their services to ensure the delivery of the best and most consistent clinical care for SMA patients. Our panel of experts recommended that patient assessment should continue either in-person, if logistically and medically appropriate, or via telemedicine or phone. Patients should have access to treating teams and clinic coordinators.
Precautions for patients with spinal muscular atrophy and their families. The panel recommended the implementation of the Saudi CDC guidelines to limit the spread of COVID-19, especially in vulnerable populations, such as SMA patients.2 These recommendations include (1) staying at home as much as possible; (2) washing hands effectively with soap or hand sanitizers with at least 60% alcohol for at least 20 seconds; (3) maintaining a distance of at least 2 meters between individuals; (4) covering mouth and nose while coughing or sneezing; (5) avoiding touching eyes, nose, and mouth with unwashed hands; and (6) routinely cleaning surfaces and tools with household cleaning products. Family members and caregivers should take all the necessary protective measures to minimize the risk of spreading infection to SMA patients.2 The panel members recommended that general measures should extend to the intervals before and after providing care to SMA patients, such as during feeding, bathing, and other everyday activities, to minimize the risk of infection. In addition to the above standard measures, SMA patients should establish a secure emergency care plan with their neuromuscular specialist and seek prompt medical attention if they or anyone in their family show symptoms of COVID-19.19
Ambulatory care services for patients with SMA. Many hospitals have suspended their outpatient clinics during the COVID-19 pandemic and replaced them with telehealth or phone services.20,21 However, the administration of SMA therapies is considered a non-elective and necessary intervention.22,23 Our experts provided the following general recommendations for SMA patients, their families, and caregivers.
Prior to hospital arrival. Patients are advised to stay home and follow all communicated measurements to limit the spread of COVID-19.24 Telehealth and phone clinics should be utilized to assess non-urgent situations. In case of emergency, patients or caregivers should call the corresponding emergency healthcare numbers so that the hospital can take the necessary steps to minimize vulnerable SMA patients’ risk of exposure to infection.25 Our panel of experts recommended that SMA patients without COVID-19 symptoms should be screened for COVID-19 using polymerase chain reaction (PCR) testing at least 72 hours prior to non-emergent procedures. Healthcare facilities should take into consideration any logistical challenges that might be considered as a burden to patients and their caregivers. In case of scheduled visits, patients should also be contacted one day prior to the visit to be surveyed regarding symptoms or recent contact with COVID-19 patients. A permit for transfer during curfew hours can be obtained through mobile applications launched by the Saudi Red Crescent. Patients and their caregivers should strictly adhere to the previously mentioned precautions for COVID-19, including the wearing of masks.
Upon arrival to treatment units. Patients should be directly admitted to the treatment room as they enter the unit. If patients need to wait upon admission, they should maintain a safe distance from all surrounding personnel.26 It is recommended that the number of supporting caregivers in the room be minimized while strictly adhering to the required precautions for COVID-19.27 Absolutely no visitors or unnecessary family members should be allowed inside the treatment room, and secure access points should be maintained. All healthcare workers performing procedures should use personal protective equipment.26,27
Follow-up after intervention. Precautions related to patient transportation to and from the hospital should be followed. After the injection procedure, all patients and accompanying family members should be educated about how to avoid COVID-19 exposure by adhering to the recommended hygiene procedures and social distancing as complementary measures even when leaving the hospital.2 Our panel of experts recommended that all post-injection follow-up visits should be scheduled virtually via clinic phone calls or video links. Caregivers are encouraged to contact and update the treating neurologist or neuromuscular specialist should the need arise.
The wellbeing of patients with SMA and their families. The rapid spread of COVID-19 and the strict measures applied by several countries, including the KSA, have applied tremendous mental stress to all community members, including families of SMA patients. Families caring for patients with disabilities are at increased risk of anxiety and depression.28,29 Treating physicians should provide regular awareness and reassurance messages along with educational materials. Physicians should advise parents and caregivers on how to maintain their mental health and wellbeing as this can impact the level of care they provide to their children or patients in their care. A calm and confident approach provides the best support for affected children and young patients.
Facility and hospital readiness for SMA management
Administration and Interruption of Spinal Muscular Atrophy Medications During the COVID-19 Pandemic. The COVID-19 pandemic has affected healthcare settings and impacted the quality of care provided to SMA patients.7 Our panel of experts agreed that SMA medications are fundamental, and their administration is considered a high priority in SMA treatment plans.30 Treating physicians are encouraged to maintain dosing schedules as planned whenever it is reasonably possible. It is expected that some patients will miss or delay their medication doses during this pandemic.7 However, our panel members highlighted that early and uninterrupted treatment is crucial to ensure better clinical outcomes, especially in infantile-onset SMA type 1. Healthcare providers should collaborate with SMA patients and their families to maintain medication administration and avoid treatment delays or interruptions.10,31,32
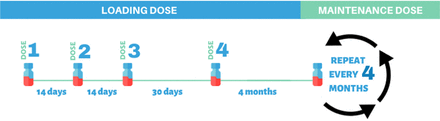
Currently, dozens of patients with SMA are treated with Nusinersen across the KSA. Nusinersen is a critical therapy for SMA patients that helps maintain a steady drug concentration in the cerebrospinal fluid and requires adhering to a scheduled dosing time. The drug is administered intrathecally through 4 loading doses over 2 months. The first 3 loading doses should be administered in 14-day intervals; the fourth loading dose should be administered 30 days after the third dose, followed by maintenance doses every 4 months (Figure 2). A missed or delayed dose of Nusinersen may lead to a decrease in cerebrospinal fluid drug exposure. Therefore, treatment delays must be avoided as much as possible for either new or currently eligible patients.7
Nusinersen loading and maintenance dosing regimen.
The United States Food and Drug Administration recommends that missed or delayed loading doses of Nusinersen should be given as soon as possible with an interval of at least 14 days between doses. In a case of missed or delayed maintenance doses, Nusinersen should also be given as soon as possible. The following doses should be continued every 4 months as per the original dosing timeline (Figure 2).33 Efforts should be made by treating physicians to reinstitute any missing doses as soon as possible. The maintenance dosing schedule is more flexible than the loading dose schedule, but adherence should be the goal.
Treatment prioritization mainly depends on the type of SMA a patient is suffering from. Type 1 patients are in more urgent need of starting treatment due to type 1’s aggressive natural history and known rapid decline. Initiating therapy for SMA type 2 and type 3 patients depends on their medical condition and the availability of resources. Treatment initiation in these SMA types should be individualized by treating physicians and MDTs by weighing (1) the risk of suboptimal treatment, (2) the rate of decline in a patient’s disease condition and respiratory functions, and (3) the risk of exposure to and contraction of COVID-19. Treatment decisions may also depend on the capacity of the hospital or healthcare facility. Capacity issues, such as limited resources, workforce, facilities, intensive care availability, and equipment, are the main factors that determine the type of treatment and selection criteria.
Onasemnogene abeparvovec-xioi is a gene therapy used to treat some SMA patients who are less than 2 years of age. It is a single intravenous infusion followed by a few hours of observation in the hospital, then one month of oral corticosteroid therapy followed by 28 days of corticosteroid tapering. This period requires careful outpatient management and laboratory monitoring.34 The decision to start gene therapy should be balanced by an MDT of experts in SMA on an individual basis.
Our panel of experts advised delaying the initiation of gene therapy in stable patients due to the risk of immune suppression by corticosteroids, which imposes a high risk of contracting infections, including COVID-19.35 In the case of gene therapy initiation, corticosteroids should be continued in SMA patients and should not be discontinued or interrupted unless discussed with the treating neurologist. Corticosteroid-treated patients and their families should take strict precautions in practicing social distancing and other recommended precautionary measures. During corticosteroid therapy, laboratory monitoring for liver functions, troponin, and platelets is recommended.34 Successful monitoring can be achieved through home blood draws and virtual clinic visits to minimize hospital exposure and possible infections.
Facility preparations related to dose administration. In general, all healthcare personnel should abide by the national infection control guidelines developed by the Saudi Ministry of Health and the Saudi CDC. As part of the routine infection control plan, procedure units in hospitals and facilities should implement policies and protocols adapted from national guidelines to reduce and prevent the transmission of infectious diseases, especially COVID-19.24
Upon arrival at the hospital, patients should be directed to the injection room immediately. All consent should be taken verbally and documented in the hospital system to avoid the written consent process. All personnel should keep a reasonable distance from each patient during all the procedure steps, including pre-procedure steps, such as informed consent collection, vital signs recording, and patient instructions. In addition, it is recommended that all personnel avoid sharing commonly touched objects like pens, telephones, and clipboards.
All healthcare workers involved in the injection procedure, including physicians and nurses, should use personal protective equipment. However, surgical masks should be worn during non-procedure times in hospitals as well.24 Triage stations should be established outside units to properly screen patients and caregivers for COVID-19 symptoms and fever before the unit entrance.36
During injection, routine aseptic techniques should be followed throughout the entire procedure.33 Minimizing the number of personnel in the IT procedure room, including healthcare staff and family members, is of the utmost priority. Healthcare personnel not involved in the procedure should avoid entering any procedure rooms occupied by patients. No medical trainees should be allowed to observe or attend procedures. This also applies to personnel switches during procedures, which should be avoided.24 However, additional medical staff should be readily available to help when needed. The alignment and readiness of the radiology team should also be considered in the case of fluoroscopy-guided intrathecal injection.
Guidance on post-injection follow-ups. While limiting encounters between medical teams and patients, our panel recommended conducting virtual multidisciplinary rounds to align treatment strategies with dietitians, pharmacists, social workers, and care-coordination staff.37
Recommendations for Multidisciplinary Team Approaches
Importance of home physical therapy exercises. Regular physiotherapy is the cornerstone of SMA management.38 Unfortunately, several physiotherapy clinics in different hospitals have limited their capacity or decided to provide physiotherapy for inpatients exclusively for the duration of the COVID-19 pandemic. Spinal muscular atrophy families are encouraged to inform their physiotherapy teams about any needs so that the physiotherapy teams can plan and communicate the best course of action.
Patients and their families are advised to continue physiotherapy support as planned per their appointment schedule via virtual clinics where physiotherapists can check their progress and address concerns and emergent issues.39 Adhering to home exercise programs can help increase core muscle strength and prevent contractures and deformities. Emerging evidence indicates that telehealth can empower patients and increase their motivation and satisfaction.37 This could be augmented with online classes for groups of patients to share their daily challenging experiences and support one another.39 In addition, documents about home exercise programs can be sent to patients. However, our panel highlighted that physical therapists should use their professional judgment to determine when and how to provide care, with the understanding this is not the optimal environment for care.
Motor functional scales assessment. Given current capacity issues due to limited telehealth resources and workforces, the focus of virtual resources should be on home physiotherapy exercise programs. Assessments recorded within 3 months prior to the initiation of therapy can be considered as a baseline assessment. Regarding the frequency of assessments, the panel members agreed that a motor functional scale assessment should be conducted upon admission for intrathecal therapy every 4 months. It is recommended that the assessment be conducted one day prior to injection to avoid frequent and unnecessary exposure to hospital settings, which could increase patients’ risk of infection.
Recommendations for home-based ventilation. Spinal muscular atrophy patients who use noninvasive positive-pressure ventilation or mechanical airway clearance devices are more vulnerable to secondary infection and at risk of transmitting infections, especially with poor fitting mask interfaces, high leak, and open ventilation systems with a tracheostomy.8,40
In conclusion, the panel of experts stressed on the importance of following national and international recommendations and guidelines in order to minimize the risk of infection. Some SMA patients are more susceptible to infection and at higher risk of contracting severe cases of COVID-19. All infected SMA patients should be closely monitored to avoid the possible rapid decline in their respiratory functions. Spinal muscular atrophy treatments should not be considered elective and should not be delayed or interrupted by the current circumstances. Early and uninterrupted treatment is crucial for better outcomes, especially in infantile-onset SMA type 1, and the dosage schedule should be maintained as planned whenever possible. The panel members also highlighted the importance of telemedicine and virtual follow-ups with SMA patients in the era of COVID-19. The importance of keeping healthcare providers, patients, and caregivers connected without overwhelming all stakeholders is a rising necessity. The use of MDTs, including respiratory care and physiotherapy, is crucial, and suitable virtual alternatives should be provided by treating physicians.
Footnotes
Disclosure. This work was supported and funded by Biologix. The authors do not have any disclosures or financial conflicts with the subject matter or materials discussed in the manuscript apart from those disclosed
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.