Abstract
Understanding seizure semiology is one of the most important and crucial steps in diagnosing a seizure disorder. Insular epilepsy may mimic other focal seizure semiologies, leading to misdiagnosis and failed epilepsy surgery. Insular seizures may begin as brief ictal symptoms, such as laryngeal discomfort and unpleasant throat sensations, and spread rapidly to the temporal or frontal regions, causing prominent ictal symptoms different to the initial insular ictal manifestation. Moreover, insular seizures are associated with complex epileptogenic networks and multiple connections. For this reason, accurate seizure semiology helps to lateralize and localize the seizure onset. The insular cortex is deep, and thus scalp electroencephalography is not always beneficial as the epileptic discharges will not be easily recorded, or they will be seen over other cortical regions like the temporal or frontal areas. Insular surgical resection is generally safe, but it requires extensive presurgical workup and surgical precautions in order to minimize mortality.
Insular seizures are a great mimicker of temporal, frontal, and parietal seizure semiology.1-3 Insular epilepsy is considered to be one of the causes of failure of temporal lobe epilepsy surgery.4,5 This concept was proposed initially by Penfield in the 1950s, and then by Isnard et al., who perform intracranial monitoring for epileptic patients with atypical temporal lobe epilepsy;4-6 they found that patients with an insular seizure onset had a unique ictal seizure semiology, including laryngeal constriction and unpleasant sensory symptoms in full consciousness.6 Subsequently, the interest in insular ictal semiology has increased, leading to the reporting of more insular ictal symptoms such as hypersalivation, dysarthria, and focal motor manifestations.7
The aim of this review is to understand insular seizure semiology, electroencephalogram (EEG) findings, surgical treatment, and outcomes in insular lobe epilepsy, in order to increase recognition of this rare seizure type. To facilitate this, electronic databases were systematically searched, including MEDLINE (PubMed and Ovid), EMBASE, and Google Scholar, to identify all published studies on insular epilepsy and semiology. Studies with a good design were included in our review. The reference lists of all potential studies were reviewed to identify additional relevant manuscripts.
Insular lobe anatomy
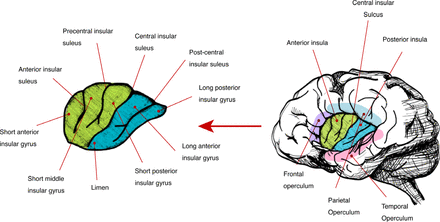
The insula was first described by Johann Christian Reil in 1809; Henry Gray called it “Island of Reil” in Gray’s Anatomy.7 More than 100 years ago, Clark was one of the first authors that discussed and described the position of the insular lobe, and called it the fifth lobe of the brain.8 The insula is a triangular folded part of the cerebral cortex, lying deep underneath the lateral cerebral fissure in both hemispheres. It is completely covered by the temporal, parietal, and frontal opercula. Despite its deep location within both hemispheres, it is considered one of the cortical components, and can be exposed by separating the upper and lower parts of the lateral fissure (Figure 1).9 A central insular sulcus divides it into anterior and posterior parts, consisting of three short gyri anteriorly and 2 long gyri posteriorly (Figure 1).10 Based on cytoarchitectonical cellular divisions, the insula can also be subdivided into 3 zones: anterior agranular, precentral dysgranular, and posterior granular.11
Insular cortex anatomy
Embryologically, the insular cortex (lobus insularis) is one of the earliest parts of the human cortex to develop, in the sixth gestational week.12 Afif et al13 divided the morphological stages of insular gyral and sulcal development into 5 stages, corresponding to the gestational age, starting from the appearance of the first sulcus at 13-17 weeks of intrauterine fetal life, to the closure of the sylvian fissure at 27-28 gestational weeks. The insula receives its blood supply mainly from the M2 segment of the middle cerebral artery (MCA). In addition to M2, the insula is also supplied by the M1 and M3 segments to some extent, and the accessory MCA from the anterior cerebral artery.14,15 The venous drainage of the insula is mainly to the deep middle cerebral vein and the superficial sylvian vein.15
Function
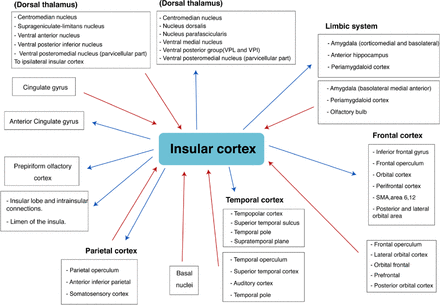
The physiological functions of the insular lobe have been extensively researched. It has been found to have special functions related to its afferent and efferent projections to various cortical and deep structures. The afferent and efferent connections of the insula are summarized in Figure 2.16 Most of the insular functions were identified by cortical stimulation and, to a lesser extent, by functional magnetic resonance imaging (fMRI), especially with regards to language function. Different studies have suggested that the insular cortex plays a major role in somatosensory, viscerosensory, cognitive, behavioral, and autonomic symptoms (bradycardia, nausea, vomiting, piloerection), as well as language and motor functions.17-20 More specifically, the anterior insula is responsible for emotional experiences and self-awareness, while the posterior insula is important for different pain modalities (pain, touch and temperature) as it receives a direct input from the thalamus.12,21,22
The afferent and efferent connection of insular cortex, this figure is adopted with permission and modification from Augustine et al 1996 (16)
Symptoms of insular cortex pathology
Pathology of the insular cortex may generate a variety of symptoms, depending on which part of the insular cortex is affected. Seizures with specific characteristics, including unpleasant somatosensory feelings, laryngeal constriction, hypersalivation, and dysphonic or dysarthric symptoms, are the most important manifestations of insular pathology.6 Seizures originating from the anterior insula may be asymptomatic at onset but may propagate and spread rapidly, leading to hypermotor symptoms, while posterior insular seizures will cause contralateral somatosensory symptoms.23
Goodkind et al24 recently published a meta-analysis on functional and other structural neuroimaging studies that showed a variety of insular abnormalities were associated with neuropsychiatric disorders such as depression, anxiety, addiction, autism, and schizophrenia.24-26 Insular lesions may also cause various cognitive, autonomic, and language dysfunctions.17,18,27 Despite the role of the insula in aphasia and language dysfunction being unclear, it has been found that language dysfunction is associated with dominant insular lesions.22 Different pathological lesions can cause insular symptoms, Chevrier et al. reviewed the structural abnormalities in 48 patients with insular/peri-insular cortex epilepsy and found neoplastic lesions in 27% of the patients, cortical malformations in 21%, vascular malformations in 19%, and encephalomalacia and atrophy in 17%.28 Moreover, other studies have reported etiologies for insular symptoms to include insular lesions such as low-grade brain tumors, vascular abnormalities including cavernomas, focal cortical dysplasia, and gliosis, the latter of which could be posttraumatic or related to an old brain insult.29,30
Seizure semiology in insular epilepsy
Isnard et al6 using intracranial monitoring with depth electrodes, were the first authors to analyze a special ictal manifestation in patients with insular epilepsy. They found that all patients had a preserved conscious level at onset. The majority of patients reported laryngeal discomfort and unpleasant throat sensations at seizure onset, and these symptoms varied in intensity, ranging from mild intensity to a strong sensation of strangulation. Insular seizure could be associated with retrosternal pain, abdominal heaviness, or shortness of breath. In addition to that, some patients reported speech abnormalities such as dysphonia or dysarthria, which progressed to a complete inability to talk for a few seconds to a couple of minutes, with associated lateralized somatosensory or somatomotor focal signs.1,6
Mazzola et al31 reviewed stereotactic stimulation of the insular cortex using stereo-electro-encephalography (SEEG), aiming to identify the seizure semiology in 222 patients admitted to an epilepsy monitoring unit for presurgical evaluation. The patients presented with either atypical temporal or insular lobe epilepsy. Ictal symptoms evoked by insular stimulation were multiple and consistent with perisylvian ictal symptoms such as somatosensory symptoms, including pain or laryngeal spasm, in addition to auditory, speech, vestibular, or olfacto-gustatory manifestations.31 Somatosensory ictal symptoms have been shown to manifest as paresthesia followed by pain and thermal sensations.32,33 Ostrowsky et al34 using insular stimulation, were the first investigators to describe pain as an insular cortex ictal symptom; the pain sensation was described as an electrical shock, burning, and painful pins and needles.34
Visceral ictal symptoms have been described as a constrictive sensation and discomfort in the laryngeal, retrosternal, or abdominal regions. In addition to these ictal visceral symptoms, patients with insular epilepsy may experience salivation as well as facial blush and, less commonly, the urge to urinate and sweaty hands.33
The insular cortex is part of a complex epileptogenic network with multiple connections to different cortical and subcortical regions (Figure 2).35 For this reason, insular epilepsy is considered to be a ‘great mimicker’, because in addition to the perisylvian manifestations, it may present with temporal or frontal lobe ictal symptoms (Table 1). Some of these temporal ictal symptoms are oral or manual automatism, and focal dyscognitive manifestations. On the other hand, frontal ictal symptoms identified in insular seizures include hypermotor or behavioral symptoms.2,36-38
Insular versus temporal versus frontal ictal clinical features.
Ryvlin et al2 using intracranial EEG monitoring, evaluated 3 patients with drug-resistant hypermotor seizures diagnosed either as non-lesional or autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). They found that the ictal onset in all three patients originated in the anterosuperior part of the insular cortex, indicating that the insula may play a major role in nocturnal hypermotor seizures.2
Dylgieri et al39 described insular ictal semiology in a pediatric age group which was characterized by autonomic manifestations, spasms, and myoclonic jerks with asymmetric atonic and hypermotor seizures. Freri et al40 retrospectively reviewed 16 pediatric patients with drug-resistant epilepsy who were suspected to have perisylvian and insular seizures, and were admitted to the epilepsy monitoring unit for presurgical evaluation. In all the patients, seizures were captured from the insular cortex. The semiology included perioral focal motor seizures associated with auditory hallucinations, unpleasant paresthesias, and epigastric rising sensations in 75% of the patients. Autonomic manifestations were noted in 50% of the patients.
Scalp EEG findings in insular epilepsy
EEG plays an important and crucial role in the diagnosis of epilepsy and seizure localization. The sensitivity of EEG in detecting epileptiform discharges depends upon multiple factors including seizure frequency, repeating the EEG, use of a prolonged recording, and performing the EEG within 48 hours of the seizure attack. All these factors will increase the detection of epileptiform discharges in patients with epilepsy.41 Given that the insula lies deep in both hemispheres and is totally covered by the temporal, parietal, and frontal opercula, it is difficult to detect interictal or ictal insular seizure activities using scalp EEG.17 The insular interictal epileptiform discharges (IEDs) may be seen over the frontotemporal, frontopolar, or mid-temporal regions.3,42
Levy et al42 reported the scalp and intracranial EEG findings in nine patients with definite operculoinsular epilepsy, confirmed by intracranial EEG, and good seizure outcomes after surgical resection or radiosurgery.The analysis of the scalp EEG in all patients revealed 14 types of IEDs. The frequent IEDs were electronegative discharges over either the frontopolar or frontotemporal and mid-temporal regions. The other types of IEDs were infrequent central or temporal discharges. None of the patients had parietal or occipital interictal discharges. The morphology of the IEDs were either spikes, bursts of low amplitude spikes, or brief low voltage fast activities (LVFA). Ten ictal scalp EEG seizures were captured using video EEG recording. Four out of these ten seizures showed no clear ictal scalp EEG changes at onset; the other 6 seizures showed a clear lateralized ictal rhythm. The ictal rhythm patterns include rhythmic spikes and waves, LVFA, rhythmic alpha activity, and rhythmic delta activity.42
Intracranial EEG findings in insular epilepsy
Intracranial EEG (icEEG) is the gold standard and most useful diagnostic study for seizure localization in drug-resistant epilepsy with atypical frontal, orbitofrontal, or temporal lobe seizures that may have originated from the insular cortex.37,42 Isnard et al. were the first investigators who describe icEEG using depth electrodes within the insula to record insular lobe seizures; it was considered a dangerous and unsafe procedure.1 After that study, several reports of icEEG monitoring of the insular cortex were published, indicating that the procedure was useful in seizure localization and in obtaining more information about insular function, using cortical mapping and electrical stimulation.3,6,39,42
Once insular epilepsy is suspected, and there is a clear hypothesis that the seizure onset may have originated from the insula, then an icEEG is indicated prior to performing epilepsy surgery, especially in cases of non-lesional epilepsy.37,43,44 There is a different approach for icEEG implantation in the insular cortex using either depth or subdural electrodes, or both.44 Despite the insular cortex being a highly vascular region of the brain, icEEG implantation is still a safe procedure if it is carefully applied. The choice to implant depth or subdural electrodes or both should be individualized to the specific patient.43-46
Levy et al42 found different icEEG seizure onset patterns in patients with definite operculoinsular epilepsy. The most common seizure onset pattern seen on icEEG is low-voltage fast activity followed by rhythmic spike waves, delta brushes, and sharp waves. Levy et al42 also found that the ictal rhythm may be seen simultaneously on the extra-insular electrodes at seizure onset in some patients. In their study, all seizures propagated and spread outside the insular cortex, which may explain the variable clinical manifestations.
Surgical outcomes in insular epilepsy
Penfield et al. and Guillaume et al. in the late 1940s were the first to attempt insular resection for intractable epilepsy.4,5,47 Unfortunately, the idea of insular surgery was initially neglected and ignored, due to a high mortality and poor surgical outcomes.48
Subsequently, a series of successful lesional insular epilepsy surgeries were published.30,49,50 Yasargil et al. reported the outcomes of 177 patients with limbic and paralimbic tumors, including 80 patients with insular tumors; they described good outcomes and no operative mortality, and more than 84% seizure freedom post-tumor resection.49 von Lehe et al51 reported a series of 26 insular lesionectomies which resulted in a seizure outcome satisfaction rate of up to 79.2% post-lesionectomy.
The decision regarding surgical resection in non-lesional insular epilepsy cases is challenging and requires adequate presurgical workup for accurate seizure localization. Malak et al52 described nine patients who underwent insular surgery due to refractory epilepsy; among them, seven patients had non-lesional insular epilepsy with an Engel Class IA outcome in six patients and an Engel Class III outcome in one patient.52 Alomar et al46 also reported a series of successful non-lesional insular epilepsy surgeries, with 33.3% of patients associated with an Engel Class I outcome, 40% with an Engel Class II outcome, 20% with an Engel Class III outcome, and 6.66% with an Engel Class IV outcome.Insular resection is generally safe, with mild and transient complications, except for some permanent motor deficits that can result from open resection of the caudal dorsal insula and adjacent parietal operculum.33,46 To avoid this possible motor complication, magnetic resonance imaging-guided stereotactic laser ablation to the dorsal caudal insula is an alternative and recommended safe procedure.33 In summary, the recent literature supports surgical interventions for lesional and non-lesional insular epilepsy, and should be considered in any drug-resistant insular epilepsy cases.
In conclusion, insular lobe seizures are an under-recognized seizure type that require special attention and careful history taking. Understanding the insular seizure semiology is a crucial and essential step in obtaining an accurate diagnosis. Insular semiological features should be correlated with the MRI and video-EEG changes, which will determine the subsequent medical and surgical treatment options aimed at obtaining satisfactory seizure outcomes.
Clinical Practice Guidelines
Clinical Practice Guidelines must include a short abstract. There should be an Introduction section addressing the objective in producing the guideline, what the guideline is about and who will benefit from the guideline. It should describe the population, conditions, health care setting and clinical management/diagnostic test. Authors should adequately describe the methods used to collect and analyze evidence, recommendations and validation. If it is adapted, authors should include the source, how, and why it is adapted? The guidelines should include not more than 50 references, 2-4 illustrations/tables, and an algorithm.
Acknowledgment
The author would like to acknowledges the efforts of the artist Dalia M. Almosa for her help in the creation of the anatomical pictures and the figure designs. The author also extends gratitude for Dr. Mohammed Abuelnor, PhD, for his comments on the anatomical section. Finally, the author would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received April 27, 2020.
- Accepted June 20, 2020.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.