Abstract
Objectives: To evaluate Epileptic drop attacks (EDAs) treatment options among pediatric neurologists in Saudi Arabia (SA) and to develop a recommendation scheme for the management of EDAs in SA. Epileptic drop attacks are one of the most pharmaco-resistant epileptic seizures. The different approaches to EDA treatment are influenced by a variety of factors, including pharmaceutical availability, costs, side effects, treating physicians’ experience and personal preferences.
Methods: This cross-sectional study was conducted online. A structured questionnaire that aimed to measure the therapeutic options for patients with EDA was electronically distributed to pediatric neurologists across SA. It contained 21 questions, and the data were collected in Excel sheets and analyzed.
Results: Our study included a cohort of 71 pediatric neurologists from SA, of which male doctors represented 60%. Most of the participating pediatric neurologists had more than 10 years of experience in the field. We found that 77% of the included pediatric neurologists used valproic acid as a first-line drug in patients with EDA. Further, in the different case scenarios provided to participants, levetiracetam, clobazam, topiramate, and rufinamide were included in the initial management protocol for EDA.
Conclusion: The majority of pediatric neurologists in Saudi Arabia chose valproic acid and/or levetiracetam as the first line of treatment for EDA. These results highlight the need for an evidence-based clinical guidelines to treat EDA.
Epileptic drop attacks (EDAs) are one of the most pharmaco-resistant and debilitating types of epileptic seizures.1,2 Also referred to as “atonic” seizures, EDAs are classified as generalized seizures by the International League Against Epilepsy (ILAE).3 Further, myoclonic and atonic-myoclonic seizures can result in EDAs, which have been identified in such epileptic syndromes as Lennox–Gastaut syndrome (LGS), idiopathic myoclonic-astatic epilepsy, and frontal and temporal lobe epilepsy.4–7 The association between EDAs and atonic seizures is important to recognize in clinical settings, because they are linked to high morbidity and injuries from falls.8 Several antiseizure medication (ASM) regimen trials have been studied, but for most patients, improvement was only partial.9,10
The LGS in children is characterized by the triad of a spike-and-wave pattern on electroencephalogram (EEG), numerous forms of seizures (tonic, atypical absence, EDAs) occurring at a high daily frequency, and poor mental development.11,12 Only a restricted number of ASMs are effective against the various seizure types associated with LGS. However, these drugs only offer partial seizure control, and they can cause serious side effects. Axial or flexor tonic spasms mainly lead to EDAs in older children and adults with LGS or with symptomatic generalized epilepsy. Thus, valproic acid is frequently advocated as a first-line therapeutic option; however, it has not been thoroughly examined in a controlled clinical study, it has complex pharmacokinetics, and it can cause hepatotoxicity and pancreatitis.13,14
In myoclonic-astatic epilepsy (MAE) of childhood, also known as Doose syndrome, seizures are classified under generalized idiopathic epilepsy. Atonia primarily affects the trunk muscles, resulting in seizure-induced falls.15–17 EDAs are produced by atonic, myoclonic atonic, or myoclonic flexor seizures in young children with MAE, one of the rare idiopathic childhood-onset epilepsy syndromes,18,19 where generalized rhythmic epileptiform discharges can be imaged via EEG in MAE. Unfortunately, treatment can be difficult, and prognosis is variable.
As a result, the approaches to EDA treatment are influenced by a variety of factors, including pharmaceutical availability, costs, side effect profiles, personal preferences, and treating physicians’ experiences. Thus, the purpose of this study is to evaluate EDA treatment options among pediatric neurologists in Saudi Arabia (SA) to develop recommendations for managing EDA in SA children.
Methods
The Saudi Pediatric Neurology Society and Saudi Epilepsy Society membership databases were used to compile a list of pediatric neurologists. In addition to physicians participating in pediatric neurology training programs and neuroscience conferences across SA, a national “neuroscience forum,” facilitated as a WhatsApp group that includes most practicing neuroscientists in SA, provided additional contact information for practicing pediatric neurologists.
A structured 21-item questionnaire aiming to measure the therapeutic options for patients with EDA was electronically sent to all certified pediatric neurologists across SA in October 2022. The questionnaire was divided into 2 sections: demographic information and clinical scenarios, where the former focused on such data as gender, age, years of practice (after board), postgraduate training, current practice, city of practice, and type of primary practice. We inquired further about the number of epilepsy and EDA patients among them. The second survey section included different case scenarios for which the pediatric neurologists could choose a treatment option. A final open-ended question was included for additional suggestions or comments. The study design and the questionnaire were approved by the biomedical ethical committee at King Abdelaziz University Hospital (reference number 177-22), all participants were given a confidential explanation of the study’s objectives and responses, and their consent was obtained.
The data were collected in Excel sheets and analyzed statistically using IBM SPSS statistics ver. 20.0 (IBM CorpArmonk, NY, USA). The variables were analyzed using the chi-square test, and descriptive analyses were conducted. Further, bar charts were used to present the data, and a level of p<0.05 was used as the cut-off value for significance
Results
Seventy-one pediatric neurologists answered our survey. Table 1 shows the demographic information, including gender, age, years of practice, city of practice, and current practice. In addition, post-graduate training and current practice type were added to the table. The neurologists were asked how often they see children with epilepsy and EDAs, and it showed that most see children with epilepsy, but less commonly those experiencing EDAs. Some pediatric neurologists stated that a drug’s availability will restrict their choices and force them to choose other medications to improve compliance. Table 2 summarizes their first-, second-, and third-line antiepileptic drug choices in the 3 different cases. Valproic acid, levetiracetam and clobazam were the first line ASMs used to treat EDA in different case scenarios. Oral steroid was preferred when steroids were considered. Followed by intravenous methylprednisolone then ACTH injections were the least to be used in Saudi Arabia. The least used ASMs were lamotrigine, clonazepam and topiramate.
- Frequencies of sociodemographic data of pediatric neurologists.
- First-, second-, and third-line antiepileptic drug choices in the three different cases.
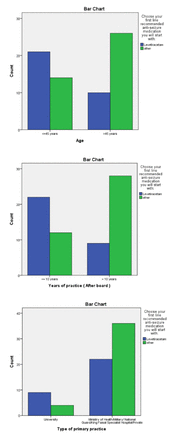
By using the chi square test, we found no significant p-value in the first and second cases and most relations could not be computed, as more than 20% of cells had counts less than 5 (small counts). However, in a child presenting with new-onset EDAs and normal initial MRI and EEG findings (third case), levetiracetam was the first-line drug of choice among 60% of neurologists aged less than 45 years and 27.8% aged more than 45 years, at p=0.006 (Figure 1a). Further, 64.7% of neurologists with less than 10 years’ experience and 24.3% of neurologists with more than 10 years’ experience chose levetiracetam as a first-line medication, at p=0.001 (Figure 1b). Another significant finding (p=0.04) concerned the type of primary practice, as 69.2% of university neurologists and 37.9% of other type chose levetiracetam as a first-line medication (Figure 1c). The other factors, including post-graduate training, current practice, and city of practice, showed no significant correlation.
- Significant correlations of first-line treatment (levetiracetam) in children with new onset epileptic drop attacks and normal initial MRI and EEG.
Discussion
The aim of this study was to determine whether pediatric neurologists in SA are recommending certain treatment options for EDAs, as well as to identify differences among the first, second, and third lines of anti-seizure medication used by pediatric neurologists in SA. EDAs are one of the pharmaco-resistant types of epileptic seizures, and because they lead to a sudden loss of postural control, they carry potential risks of falls and serious injuries to patients, especially after 2 years of age, when children can sit, stand, and walk.2
The EDAs can be isolated or can present in the context of an epileptic syndrome, such as LGS, myoclonic atonic seizures (Doose syndrome), and others. These seizures are often noticeably short in duration and involve a complete loss of awareness; they manifest as atonic, tonic, or, sometimes, myoclonic seizures.2,20
We presented 3 different case scenarios, the first concerning LGS, the second a child with a clinical picture of Doose syndrome, and the third isolated EDAs. In our study, the most commonly used ASM in pediatric patients with mixed epileptic syndromes were valproic acid, levetiracetam, clobazam, clonazepam, topiramate, and rufinamide, where the former represents one of the first lines of treatment for LGS.20 On other hand, the responses (>50% seizure reduction) to the first 3 ASMs were 26% for levetiracetam, 17% for valproic acid, 31% for other ASDs, and 26% combined in those with myoclonic atonic seizures (Doose syndrome).21
Our study was conducted among 71 pediatric neurologists across SA who were asked about three different case scenarios of common pediatric patients who frequently visit the pediatric neurology clinic with mixed types of epilepsy, including EDAs, and we found that 77% of the included pediatric neurologists used valproic acid as the first line of treatment in patients with EDAs, many of whom practice in the capital city of Riyadh and the others in different regions of the country. However, levetiracetam and clobazam were the next drugs of choice after valproic acid, considered second-line treatments by some neurologists.
Other ASMs may be considered and included, such as lamotrigine, topiramate and rufinamide, which can be efficacious in treating patients with mixed epileptic types, such as LGS, and which is considered one of the three most effective lines of treating EDAs.21,22
While most of pediatric neurologists practicing in SA were more likely to use valproic acid as a first-line treatment, in a child who presented with new-onset EDAs and normal initial MRI and EEG findings (third case), levetiracetam was the first drug of choice among 60% of neurologists aged less than 45 years of age and among 27.8% aged more than 45 years. Further, this study found that 64.7% of pediatric neurologists with less than 10 years’ experience and 24.3% with more than 10 years’ experience chose levetiracetam as a first-line medication. Finally, 69.2% of university neurologists and 37.9% of other types chose levetiracetam as a first-line medication.
Pediatric neurologists with many years of clinical experience were more likely to select levetiracetam as their first choice, reflecting its better efficacy, confirmed by these neurologists’ longer practice and experience. In addition, the side effect profile of valproic acid remained a concern for many pediatric neurologists, contributing to the choice of levetiracetam as a safer option.
Peripheral hospitals may have more limitations in terms of availability compared to larger tertiary care hospitals, which may explain the significant percentage of neurologists who use other ASMs as a first-line treatment instead of valproic acid. On the other hand, clobazam and clonazepam was commonly used despite their side effect profiles.
Pediatric neurologists were also asked about using a steroid as a first-line treatment in patients, and most found it non-applicable. Another reason for not selecting steroids is that methylprednisolone, or ACTH, has variable side effects and lacks availability, which may explain the regional differences in its use.
A paper published in Porto Alegre, Brazil, in 2011 focused on the long-term control of EDAs with a combination of valproate, lamotrigine, and benzodiazepine as a “proof of concept,” open-label study.23 The results showed the significant potential of this combination in significantly reducing or completely controlling EDAs in patients with symptomatic epilepsy. The fact that the effect was sustained for a long period and that most patients tolerated the ASM means this regimen could be replicated by pediatric neurologists in SA. Care monitoring and regular follow-up are mandatory for serious side effects because of the risk of SJS, which developed in one patient.23 Options like valproate, lamotrigine, and topiramate were considered to be the first-line drugs in other studies.24 Other ASMs options like rufinamide, and zonisamide are considered effective to alleviate EDA especially in epileptic encephalopathies like LGS.25 However, due to availability issues in Saudi Arabia, they were not one of the initial treatment options. Steroids in different formulations are considered effective modality. Up to 46% of children with epilepsy including EDA can show improvement after a course of steroid.26 In our study, steroids (mainly oral steroid) were preferred to be used in EDA, however, it was after the 3rd option as ASM. Though corticosteroids are used to treat different forms of epilepsy, it is considered a second and a third line option due to their side effects and tolerability.27 Other effective interventions are ketogenic diet, corpus callosotomy and vagal nerve stimulation.28
In conclusion, there is some agreement in the choice of ASMs for EDAs among pediatric neurologists across SA, with most choosing valproic acid or levetiracetam as a first line of treatment to control EDAs with a minimal side effect profile, good tolerance, and favorable long-term control in regular follow up. Followed by clobazam due to its potential efficacy. While a minority choose other ASMs, such as clonazepam, lamotrigine and topiramate. The study suggests that if valproic acid or levetiracetam fails to control EDAs, then the addition of clobazam, lamotrigine, and topiramate should be considered. In the era of precision medicine, future disease-specific therapies well improve the prognosis in such conditions. Finally, the study could be limited by the small number of participants, however, the number of Pediatric Neurologists in the country is small compared to other specialties. This study shed light on the management of a debilitating type of seizures. Hopefully, it can guide future precision-guided treatment for EDAs.
Acknowledgment
We would like to acknowledge the assistance and cooperation of all pediatric neurologists who participated in the study, particularly members of the Saudi Neuroscience Forum. Without their cooperation, honest input, and support, this study would not have been possible. Also, the authors would like to thank Scribendi company (https://www.scribendi.com) for English language editing
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received January 24, 2023.
- Accepted June 13, 2023.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.