Abstract
Objective: To study the possible relationship between amyotrophic lateral sclerosis (ALS) patients and their susceptibility to serum ferritin level elevation.
Methods: We searched the PubMed, Springer, Medline, and OVID databases for any-language original research articles relating to serum ferritin levels in ALS patients published between June 2005 and June 2015. The search term used with ‘amyotrophic lateral sclerosis’, ‘ferritins’, ‘ferritin’, ‘iron’, ‘iron stores, ‘iron status, ‘iron intake’, and ‘iron consumption’. The meta-analysis software RevMan 5.0 was used for the heterogeneity test, and to test for the overall effect.
Results: Six case-control studies met our inclusion criteria including data from a total of 1813 participants. The mean difference of serum ferritin levels comparing ALS to healthy controls was 69.05 (95% confidence interval: 52.56-85.54; p<0.00001); heterogeneity: p=0.03; I2=50%. The findings indicate homology in the sensitivity analysis. Funnel plot assessment indicated publication bias.
Conclusion: Our results suggest that ALS is positively associated with susceptibility to the elevation of serum ferritin levels; however, further evidence is required to support this.
A myotrophic lateral sclerosis (ALS) is a neurodegenerative disorder caused by the loss of motor neurons leading to progressive bulbar, limb, and respiratory muscle weakness.1 Death generally occurs within 2-5 years after symptom onset, mostly due to respiratory failure.2 Prognostic factors related to shorter survival time include older age, short time from symptom onset to diagnosis, bulbar onset,3 lower uric acid (UA),4 albumin, and creatinine levels.5 Oxidative stress, glutamate induced excitotoxicity, mitochondrial dysfunction, inflammation, and disturbed metal metabolism has been proposed as the causes of neurodegeneration in ALS.6 Iron metabolism has been implicated in the pathogenesis of several neurodegenerative disorders; for example, Alzheimer disease (AD), Parkinson disease (PD), Huntington’s disease (HD), neurodegeneration with brain iron accumulation (NBIA), restless leg syndrome (RLS), Friedreich’s ataxia (FRDA), and neuroferritinopathy due to its ability to generate cytotoxic reactive radicals.7 Using histological methods, Kwan et al8 showed that iron was increased in the motor cortex in 2 ALS patients. Early confirmed diagnosis, oral riluzole, which is the only drug approved for treatment, better hospital symptomatic care, early use of nutrition, and respiratory management have shown an improvement in survival.9 The ALS Functional Rating Scale (ALSFRS) and % forced vital capacity (%FVC), have been shown to decrease linearly over time and predict survival.10
In the current meta-analysis, we aim to analyze the findings of published original research articles investigating the relationship between ALS and susceptibility to serum ferritin level elevation compared with healthy controls.
Methods
Search strategy
We systematically searched PubMed, Springer, Medline, and OVID for original research articles, scanned the reference lists of identified articles, and conducted hand-searching of relevant journals according to the Cochrane handbook to retrieve published studies on serum ferritin levels in ALS patients. We used free text and the medical subject heading (MeSH) terms ‘amyotrophic lateral sclerosis’, ‘ferritins’, ‘ferritin’, ‘iron’, ‘iron stores, ‘iron status, ‘iron intake’, and ‘iron consumption’. The search period was all-inclusive between June 2005 to June 2015, no language restrictions were added.
Study selection
We included all observational studies in which the ALS patients were diagnosed serially with definite or probable ALS according to diagnostic criteria for definite or probable ALS based on the El Escorial World Federation diagnostic criteria. The exclusion criteria were as follows: 1) No original research (reviews, editorials, non-research letters); 2) case reports and case series; 3) studies concerning children, adolescents, and pregnant women; 4) study subjects with hypertension, hemochromatosis, gout attacks, chronic liver disease, liver cirrhosis, or chronic renal diseases, chronic inflammatory diseases, a percutaneous enterogastrostomy, iron, uric acid- or lipid-lowering medications; 5) test for the concentration of ferritin-L subunit.
Data extraction and quality assessment
Two meta-analysis trained investigators independently reviewed the search results and selected articles to determine eligibility and to extract study data. To assess study quality, we used the Downs and Black quality assessment scale (DBQAS) of case-control studies. Each of the criteria were categorized as clearly yes or clearly no. A score between 0 and 30 was assigned to allow for quality analysis (0 denoted noncompliance with any criteria, and 30 denoted fulfillments of all criteria).
Data synthesis and statistical analysis
Heterogeneity was quantified with the I2 statistic, which describes the proportion of total variation in the study estimates that is due to heterogeneity. A meta-analysis of 6 studies comparing serum ferritin levels between ALS patients and healthy controls was performed. All statistical analyses were performed using RevMan 5.0 (Copenhagen: the Nordic Cochrane Centre, the Cochrane Collaboration, 2008), and included an integrated analysis as follows: For mean difference (MD) estimates from individual studies, we used an inverse-variance weighted fixed-effect model when the heterogeneity test was p≥0.05, and a random-effect model when the heterogeneity test was p<0.05. We used sensitivity analyses to assess the relative influence of each study. Finally, we assessed publication bias using funnel plots.
Results
General characteristics of the ALS patients
Six case-control studies11-16 published between 2008 and 2015 were included in the meta-analysis and comprised 1053 cases of ALS (male to female ratio = 1.40), and 760 healthy control cases from the UK, USA, France, and Japan. The characteristics of the 1053 ALS patients are summarized in Table 1. The mean age at the sampling time of ALS patients, which was recorded in 5 reports, was 61.9 years. In one report,12 the mean age at symptom onset was 56.3±13.0 years. The mean percentage of bulbar onset, which was defined as symptoms first occurring at the bulbar level with dysphagia, dysphonia, or dysarthria was 32.8%. Only one report13 reported the body mass index (BMI) at 22.6 kg/m2. The mean ALSFRS of 3 reports,13-15 was 32.5, and the mean %FVC of 2 reports13,15 was 87.8%. The DBQAS scores of studies included were 26-29 points (Table 1).
General characteristics of included amyotrophic lateral sclerosis (ALS) patients.
Meta-analysis results of the serum ferritin level in ALS patients
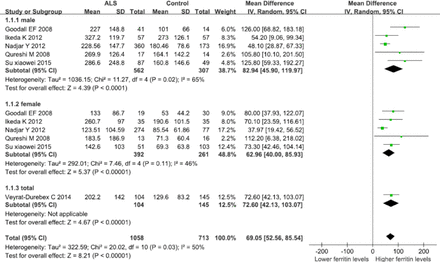
There was heterogeneity when comparing serum ferritin levels of male ALS patients to healthy controls (p=0.02; I2 =65%), so the studies were analyzed using the random-effect model. The MD of serum ferritin levels compared between ALS patients and healthy controls was 82.94 ug/L (95% confidence interval [CI]: 45.90-119.97; p<0.00001) (Figure 1). There was no heterogeneity when comparing serum ferritin levels of female ALS patients to healthy controls (p=0.11; I2 =46%), so the studies were analyzed using the fixed-effect model. The MD of serum ferritin levels compared between ALS patients and healthy controls was 62.96 ug/L (95% CI: 40.00-85.93; p<0.00001) (Figure 2). There was heterogeneity when comparing the serum ferritin levels of all ALS patients with healthy controls (p=0.03; I2 =50%), so the studies were analyzed using the random-effect model. The MD of serum ferritin levels compared between all ALS patients and healthy controls was 69.05 ug/L (95% CI: 52.56-85.54; p<0.00001) (Figure 1).
Forest plot of relationship between ALS and susceptibility to serum ferritin level elevation analyzed by the random-effects model. Note: Studies are divided by gender (male, female, and both genders). Diamonds represent mean difference (MD) estimates from inverse-variance (IV) weighted random-effects model. Unit of serum ferritin levels: ug/L, CI - confidence interval
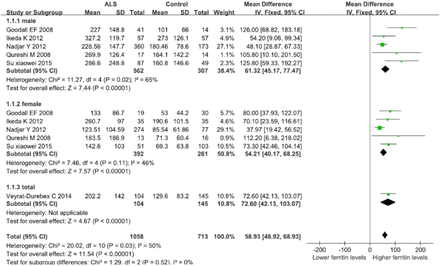
Forest plot of relationship between ALS and susceptibility to serum ferritin level elevation analyzed by the fixed-effects model. Note: Studies are divided by gender (male, female, and both genders). Diamonds represent mean difference (MD) estimates from inverse-variance (IV) weighted fixed-effects model. Unit of serum ferritin levels: ug/L, CI - confidence interval
The sensitivity analysis
The overall effects were analyzed using a fixed-effect model to include 6 studies comparing the serum ferritin levels of all ALS patients with the healthy controls. The MD of serum ferritin levels compared between ALS patients and healthy controls was 58.93 (95% CI: 48.92-68.93; p<0.00001) (Figure 2). The results were the same as the random-effect model, indicating the reliability of the results.
Publication bias
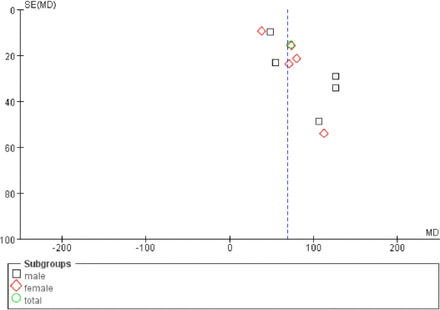
Funnel plots were drawn using RevMan 5.0 for the susceptibility of ALS patients to serum ferritin level elevation. The funnel plots were not symmetrical and funnel shaped, indicating publication bias (Figure 3).
Funnel plot of relationship between ALS and susceptibility to serum ferritin level elevation analyzed by the random-effects model. Note: The funnel plots indicate the existence of publication bias. MD - mean difference, SE - standard error.
Discussion
In the present study, we conducted a meta-analysis to summarize the independent positive association between ALS patients and their susceptibility to serum ferritin levels. Recent studies indicated that patients with acute ischemic stroke,17 neurodegenerative diseases (AD, PD, HD, FRDA, aceruloplasminemia),18 and diabetes mellitus peripheral neuropathy had high serum ferritin levels. Abril-Ulloa et al19 suggested that high ferritin blood concentrations were related to the presence of metabolic syndrome (including abdominal obesity, insulin resistance, hyperglycemia, hyperlipidemia, and hypertension), which was a significant risk factor for cardiovascular disease, type 2 diabetes, and cancer. In contrast, low serum ferritin levels were reported in restless leg syndrome and neuroferritinopathy patients.7,18 The role of ferritin in these conditions may relate to oxidative stress.7,17-19 The serum ferritin levels in ALS were statistically higher compared with healthy controls in our study. The physiological mechanisms and effects of increased iron deposition in the motor cortex in ALS patients remain unclear.8 Disorder of iron ion homeostasis in motor neurons or microglia could cause disease, especially in activated microglia where accumulated iron could gradually produce iron overload induced neurodegenerative diseases.20
Ferritin is an iron storage protein consisting of heavy (H) and light (L) subunits, associated with responses to oxidation stress and iron storage.7 Because ferric iron (Fe3+, Fe2+) causes a catalytic Haber-Weiss reaction (O•2−+ H2O2→O2+•OH+OH−), it is essential for the maintenance of Fe homeostasis to maintain a healthy brain, and Fe-related neurodegenerative disorders can result from both iron accumulation in specific brain regions or defects in its metabolism and/or homeostasis.18 H-ferritin is mostly distributed in neurons, L-ferritin in microglia, and both H- and L-ferritin in oligodendrocytes.20 Neuroferritinopathy is the indirect model to study the connection between ferritin and neurological disorders as an autosomal dominant extra-pyramidal movement disorder caused by mutations in the ferritin light chain gene with the characteristics of iron deposition in the basal ganglia and cavitations.7 Iron over deposition in the cerebral cortex, cerebellum, and basal ganglia can result in neuronal loss through oxidation stress and the inflammation process.21 Based on this, the use of iron chelating therapy to reduce serum ferritin levels in ALS may have therapeutic benefits and improve survival time.
The limitations of this meta-analysis are related to high heterogeneity (Figure 1), the lack of original research reports (Table 1), and publication bias (Figure 3). The elevated heterogeneity and publication bias may be explained by the study design, type of measure of association, geographic area, ferritin assay technique, %Bulbar onset, age at sample, ALSFRS, BMI, %FVC, non-invasive positive-pressure ventilation, oral riluzole, and enteral nutrition.1 Because serum ferritin is an acute-phase reactive protein that can be raised in the presence of acute or chronic inflammation, it changes according to the inflammation levels of ALS. Dysphagia is a common feature in ALS patients with 32.8% prevalence at disease onset (Table 1), with progression during the course of its natural history leading to progressive hypophagia, dehydration, lung infection, protein-energy malnutrition, and turns the respiratory function from bad to worse. This may elevate the serum ferritin levels according the mechanism of ferritin in inflammation. In 2 papers13,15 reported that the ALS-FRS and %FVC declined year after year in natural history of ALS. Ikeda et al13 also reported that the ALSFRS and %FVC rapid worsening (ALSFRS decline ≥1 point/month and annual decline of %FVC ≥30%) had significant positive association to the baseline levels of ferritin. There is still a lack of research exploring the relationship between decreasing ALSFRS and %FVC and serum ferritin level elevation instead of the baseline ferritin levels. There is a lack of research exploring the relationship between decreasing ALSFRS and %FVC and serum ferritin level elevation. Nemba et al22 showed us that lower BMI and serum insulin-like growth factor 1 (IGF-1) could increase serum ferritin levels in malnourished adolescents with eating disorders.1 Only one report13 picked up the BMI value, we found that the changes of BMI value have relationship with serum ferritin levels. The BMI may be one of causes of publication bias. So, we have to talk on this phenomenon.
Iron overload was defined as serum ferritin levels greater than 200 ug/L for males, and 150 ug/L for females by the World Health Organization (WHO).19 Five studies11-14,16 reported serum ferritin levels indicating iron overload in males, but only 2 studies12,13 reported iron overload in females. The mean serum ferritin level of female healthy controls in one study13 was 190.6 ug/L, which is higher than the upper limit of iron overload. The mean serum ferritin level was reported in both genders of another study15 as 202.2 ug/L, which is higher than the iron overload for males.
Elevated serum ferritin levels could potentially influence developing ALS and survival time due to the important role of ferritin in the physiological mechanisms of ALS.16 In the early stages of this disease, iron-chelating therapy to reduce serum ferritin levels may have a positive effect on ALS survival time.
In conclusion, the results of the present meta-analysis indicate that high serum levels of ferritin are positively associated with ALS. Additional prospective studies are needed to confirm if high serum ferritin is a valid biomarker of ALS, which can be used to identify pathological cut-off values.
Footnotes
Disclosure
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- Received September 9, 2015.
- Accepted November 25, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.