Abstract
Objectives: To determine the effects of sensorimotor integration training on postural control in Parkinson’s disease.
Methods: This prospective, randomized controlled trial was conducted at Hacettepe University (Ankara, Turkey). The study was carried out from August 2012 until March 2015 and included 24 Parkinson’s patients with stage 2–3 according to the Modified Hoehn&Yahr Rating Scale. The patients were divided into 2 groups (control and study). The control group received conventional physiotherapy; the study group received sensorimotor integration training combined with conventional physiotherapy, 2 times per week for 6 weeks. We assessed the patients with clinical balance tests and computerized dynamic posturography. Assessments were performed at baseline, 7- and 12-weeks follow-up.
Results: Computerized dynamic posturography posturography values (5th and 6th positions, composite balance, and vestibular system scores) were higher in the study group than in the control group. The improvements were maintained at the 12-week follow up except 6th positions scores (p<0.05).
Conclusions: Sensorimotor integration training combined with conventional physiotherapy approach ameliorated postural control by improving vestibular system in patients with Parkinson’s disease by improving sensory processes.
Postural instability is a symptom of Parkinson’s disease (PD) that causes severe disability.1 It involves a loss of postural control. The pathophysiology of postural instability in PD is complex and multi-factorial. Poor and slow anticipatory postural responses, inadequately organized automatic postural reactions, defective somatosensory integration and modulation of afferent sensory information, orthostatic hypotension, age-related sensory and postural changes, rigidity, and other Parkinsonian signs can occur in postural instability, resulting in an increase in its severity.2-3 Postural instability is poorly responsive to medications containing L-dopa. Therefore, other therapies, such as physiotherapy, have come into prominence.4 Many different modes of physiotherapy intervention can be used to decrease postural instability. This includes classical balance training, external cueing training, and movement strategy training.5 The most commonly used treatment is balance training.3,6 However, the pathophysiology of postural instability suggests that the therapeutic program for postural instability should be complex and multifaceted.
The sensory integration training approach was devised by Jean Ayres to “take in, interpret, and integrate the spatial temporal aspect of sensory information from the body and the environment to plan and produce organized motor behaviors”.7 The approach usually used in children emphasizes the use of sensory and perceptual components to produce motor responses.8-9 The multi-sensory aspect of this approach could contribute to a decrease in postural instability in PD patients when considering the pathophysiology of postural instability. Sensorimotor integration training (SMIT) was created to keep in mind the principles of sensory integration training and the mechanisms of postural instability in PD. A pioneering study that we previously completed with fewer participants and no follow-up results has shown that SMIT has improved some parameters in Parkinson’s patients.10 Therefore, we planned this study was to investigate the short and long time effects of SMIT on postural instability in PD patients.
Methods
Patients
This randomized controlled trial was conducted at Hacettepe University (Ankara, Turkey). The study was carried out from August 2012 to March 2015, and it was approved by the local ethical committee of Hacettepe University (FON 12/26-5). The study was according to principles of Helsinki Declaration.
The inclusion criteria were as follows: patients diagnosed by a neurologist with idiopathic PD, scores of between 2 and 3 on the Hoehn Yahr Staging Scale (HYSS) and at least 26 points on the mini mental test,11 ages of at least 50, no other neurologic diseases, and no changes in medications (levodopa/carbidopa and standard-release dopamine agonists) or dosages during the course of treatment. Patients with severe mental or psychological disorders were excluded.12 Additionally, patients who had participated in a physiotherapy program within the last 6 months were excluded.
Patients who had read and signed informed consent forms were randomly divided into study and control groups using a computerized random number table. Baseline assessments were made one week prior to training, and second measurements were obtained during the first week after the end of the 6-week training period. Follow-up measurements were carried out 6 weeks after the last session (at the 12th week). After the 6-week training period, all patients were asked to continue their normal daily activities and home exercises until their assessment at the 12th week. The evaluations were made during the “on” period.
Measurements. Clinical Measurements
The Unified Parkinson’s Disease Rating Scale (UPDRS) provides information about the severity of the disease and uses a point-scoring system ranging between 0 and 4 for each section.13 In our study, we used the sections assessing motor and daily life activities.
The Timed Up and Go Test (TUG) is one of the functional tests used to evaluate the dynamic components of balance.14 We repeated the test 3 times and used the mean values of the measurements. The Functional Reach Test (FRT) measures a person’s ability to maintain an upright position while being subjected to perturbations. It yields information about proactive and adaptive postural control.15 The Berg Balance Scale (BBS) is a functional scale assessing static and dynamic postural control associated with 14 different activities.16
Laboratory measurements
Computerized Dynamic Posturography (CDP) (Neurocom Smart Balance Master Systems, USA) is valid and reliable for measuring postural control, providing objective information. The system consists of a movable support surface within a movable surrounding (enclosure) consisting of an overhead support bar with a patient harness to prevent individuals from falling. The CDP includes many different tests, such as the Sensory Organization Test (SOT) and the Adaptation Test and Motor Control Test. The SOT protocol includes 6 different test positions in which the proprioceptive, visual, and vestibular systems are assessed.17 The data calculation for the SOT includes the composite balance score (CBS) and an analysis of sense. Sensory analyses detect functional losses in sense perceptions and/or abnormal sense priority.
Interventions
Individuals in the control group received a classic 6-week physiotherapy program with 1-hour sessions 2 a week. The individuals in the study group received 30-minute SMIT in addition to the classic physiotherapy program during the same session 2 a week in the first 6 weeks.
Classic physiotherapy program
This program was individually tailored and included flexibility, strengthening, posture, breathing balance, walking exercises, and other functional activities. Balance training was one of the most important components of this program, and the balance training protocol described in Cattaneo et al.18 was performed in this study. The training programs progressed and intensified each week, depending on the individual’s performance, tolerance, and needs.
Sensorimotor integration training
We stimulated the patients’ proprioceptive, visual, and vestibular systems using appropriate methods. Considering the visual dependency of PD patients,19 exposure to visual stimuli was initially preferred. In the early stages of training, visual stimuli were chosen as the first sensory cue to improve balance via enhancing the body image, practicing regular and coordinated movements, or achieving postural changes required for movement and postural regulation. The training program was then redesigned to predominantly stimulate the most affected systems according to the results of the sensory analysis derived from the SOT. Visual stimuli were reduced, and the sensor motor integration training focused on using the other 2 sensory systems. When the intended responses were achieved for each system, the training was continued by combining the senses to enable modulation and a combination of sensory information in the higher brain. Furthermore, the exercises were redesigned to include the motor components of postural control and to improve sensory-motor-perceptual integration. To achieve this, we created a special “walking trail.” During patient training on this trail, principles associated with motor imaginary, body awareness, and auditory cues were used. Patients were encouraged to make decisions, adjust postural preparations, and decrease mistakes on the walking trail. These challenges became progressively more difficult as patients’ performance improved10 (Table 1).
Sensorimotor integration training program.
Statistical analysis
We selected a p-value of 0.05 for statistical significance. Statistical analysis was performed by the IBM SPSS Statistics for Windows version 17.0 (SPSS Inc, Chicago, IL, USA). Physical characteristics were assessed using the chi-squared test for gender and the Mann-Whitney U test for age and disease status. We used the Wilcoxon Signed Rank Test for intragroup comparisons and the Mann-Whitney U test for inter-group comparisons. The pre-therapy values were subtracted from the post-therapy values and follow-up values to calculate differences, which were then used for intergroup comparisons using the Friedman test to determine whether there were any differences among the pre-therapy, post-therapy, and follow-up datasets. In cases where there was a difference, the Wilcoxon Test was used to determine which values were most significantly different. For both groups, we calculated Cohen’s d values for posturography (effect size) to determine the intragroup efficiency of the therapy. The efficiency of the therapy was accepted to be high if Cohen’s d value was equal to or larger than 0.8. Meanwhile, the efficacy was assumed to be of medium strength if Cohen’s d value was between 0.5 and 0.8.20 The confidence interval was calculated for all disease severities and postural control values.
Results
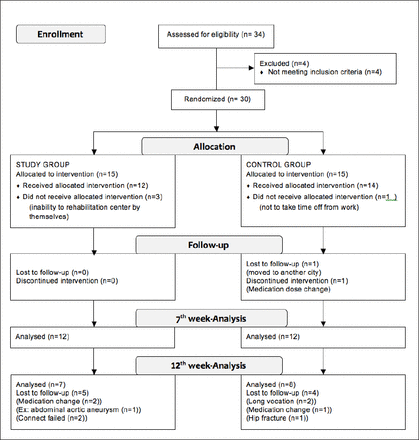
We evaluated 34 patients with PD. Of these, 30 were initially enrolled, but six did not complete the study. The remaining 24 patients who completed the 6-week training period were assessed. Ten patients dropped out for various reasons during the follow-up period, and 15 patients were eventually assessed at the 12th week (Figure 1). The demographic and clinical characteristics of the patients are shown in Table 2.
Participant flow diagram for participant inclusion, allocation, follow-up, and analysis.
Demographic and clinical characteristics of the groups.
Treatment Results
The UPDRS values (motor p=0.930, activities of daily living (ADL) p=0.113, and total p=0.297) of both groups were similar.
According to the clinical balance tests (FRT p=0.024, BBS p=0.027, and TUG p=0.027), the patients in the study group showed more improvements after treatment. The SOT values (fifth p=0.005 and sixth positions p=0.001, CBS p=0.0001, and vestibular system scores p=0.001) of the study group were significantly higher than those of the control group (Table 3).
Disease severity and postural control values of groups.
Follow-up results
The study group showed improvements in all clinical balance tests, while the control group demonstrated significant improvements in only the motor-UPDRS and FRT values. When we compared the 2 groups, the BBS (p=0.037) and TUG (p=0.002) were higher for patients in the study group, while the 2 groups were similar with respect to the UPDRS (motor: p=0.861, ADL: p=0.352, total: p=0.352) and FRT (p=0.115) scores.
According to the SOT values, the changes in the fifth and sixth positions, CBS, and vestibular system scores of the study group were unchanged. The control group’s values remained unaltered. When we compared the 2 groups, the fifth position p=0.027, composite balance p=0.042, and vestibular system scores p=0.048 were higher in the study group (Table 3).
Discussion
The most important result from this study was that, in addition to classical physiotherapy, SMIT improved the patients’ postural control by increasing their utilization capacity for vestibular information. The literature shows different results regarding the effects of balance training on the UPDRS score. Marchese et al21 found improvements in this scale after cued-balance training, while Smania et al22 reported that the UPDRS scores did not change despite improvements in a patient’s balance. In our study, the UPDRS scores improved in 2 groups, and the groups were similar by the end of the study at follow-up. The improvement in the 2 groups may be related to our comprehensive conventional training program. Furthermore, the UPDRS scores of the two groups may be similar because the UPDRS is less sensitive to relatively small changes in postural control.
In our study, postural control was assessed using clinical tests and the SOT. The TUG, BBS, and FRT include dynamic transition activities that make postural stability more challenging.23 Complex integration between different control mechanisms is required during these activities. TUG and BBS include many activities used in daily life. For patients, the “up from the chair” activity is the most time-consuming stage of the TUG, and their balance may be shifted backwards because they are unable to move their body forward as a result of insufficient displacement and speed. Thus, patients are not able to bear weight on their forefoot. Therefore, the FRT is a difficult test for PD patients. The BBS is a more comprehensive evaluation, although it includes similar activities as the TUG and FRT. The improvement in BBS following balance training has already been shown in previously published studies, which were methodologically different from our study. The TUG values improved less than those using the BBS, whereas the FRT values did not change at all according to various studies using standard balance training.23-25 These results suggest that standard balance training is insufficient for improving FRT values. In the present study, the BBS, TUG, and FRT scores improved in the study group relative to the control group. Similarly, SOT values in the study group improved more than in the control group. Studies in the literature have shown that the third, fourth, fifth, and sixth positions scores, in addition to the CBS, vestibular system, and visual system scores are lower in PD patients than in healthy individuals.26 In our study, we found improvements in the fifth and sixth position scores and the CBS and vestibular system scores. Some studies, which were methodologically different from this study in terms of the control groups, applications, and follow-up, reported similar SOT results as in our study.27-29 However, the improvement in SOT values in our study were clearly higher than the improvements in these other studies.
Our results indicate that there were significant differences between the groups with respect to postural control. Sensory inputs intensively used in the study group may be one of the reasons for these differences. The use of vestibular inputs was an important part of the SMIT. The vestibular system provides information about head and neck movements and regulates them via the vestibulo-ocular, vestibulo-collic, and vestibulo-spinal reflexes.30-32 This system has important connections with the cerebellum, which modulates multiple sensory inputs and plays a fundamental role in postural control by managing vestibulo-spinal reflexes and contributing to motor learning, perception, postural control, and orientation.33,34 In the light of this, we believe that the SMIT may engage the vestibular system, cerebellum, and their downstream pathways. Training on the walking trail in the SMIT protocol appeared to be one of the most effective factors at play because this exercise included the use of various multisensory approaches, such as motor imagery, auditory cues, and multisystem integration. Sehm et al35 reported that there were gray matter changes in the cerebellum and parietal, premotor, and anterior cingulate cortexes after balance training, and their findings support our hypothesis regarding the cerebellum and its pathways.
We expected an improvement in proprioceptive system position scores following SMIT. Instead, we only observed improvements in vestibular system scores, but no changes in proprioceptive system scores. Considering the complex sensory structure associated with postural control, changes in this structure might not be solely attributed to vestibular system enhancement. Due to the patients’ high baseline scores, we could not demonstrate proprioceptive improvement. Nevertheless, we think that using intensive proprioceptive information in sensory organization training might indirectly contribute to increased postural control.
Our 6-week follow-up assessments revealed that improvements in the BBS, TUG, fifth position, CBS, and vestibular system scores were maintained in the study group. These results suggest that the positive effects of the SMIT are maintained for long durations. This may mean that the compensatory effects of the cerebellum on postural stability can be maintained after a combination treatment.
Study limitations
Our study had some limitations, the most important of which was our small sample size. We calculated the power of our study (28%) using the CBS after analyses made by comparing the post-therapy values of both groups. The intergroup effect size was 0.558. Each group should include 47 patients for 80% power with this effect size and a p-value of 0.05; however, it may be difficult to attain these numbers in clinical settings. On the condition that the effect size was calculated using the pre-treatment and post-treatment values (Cohen’s d correction: dcorr = dposttest – dpretest),36 the power of the study was 81%. The intragroup power of the experimental group was 99%. Another limitation of our study was the small number of patients who attended the 12-week follow-up assessment. This made it difficult to draw conclusions about the maintained development rate. Therefore, further work is needed about efficacy of SMIT in PD.
In conclusion, combining a classical physiotherapy program with the SMIT may be a successful approach to treatments geared toward decreasing postural instability and improving balance in PD patients. The vestibular system responds rapidly to treatments in patients with PD. Interestingly, the cerebellum is relatively unaffected even though it plays a part in motor learning, which should be considered when planning physiotherapy programs.
Acknowledgments
The authors would like to thank those people with Parkinson’s disease who participated in this research and TUBITAK (The Scientific and Technological Research Council of Turkey) for doctoral scholarship (it was not specific for the research).
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received January 14, 2018.
- Accepted May 2, 2018.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.