Abstract
Objectives: To perform the Turkish translation, reliability, and validity study of the PedsQ™-3.0 Multidimensional Fatigue Scale (PedsQL-MFS) in patients with Duchenne Muscular Dystrophy (DMD).
Methods: This prospective, cross-sectional, observational study was held in Hacettepe University, Faculty of Physical Therapy and Rehabilitation between January 2016-August 2018. Turkish translation of the PedsQL-MFS was conducted based on the steps addressed in the translation manual of the original research. The psychometric features of the Turkish version of PedsQL-MFS including feasibility, internal consistency, and test-retest reliability, construct, and criterion-related validity as well as parent/child agreement were investigated on a total of 71 children and their parents.
Results: The mean age of boys with DMD included in the study was 102.94±23.23 months with a mean 17.15±2.98 BMI. Internal consistencies of Child Self Report General Fatigue, Sleep/rest Fatigue, and Cognitive Fatigue items were 0.74, 0.65, and 0.83 while, 0.89, 0.84, and 0.91 in Parent Proxy Report. The ICC values of Child Self Report and Parent Proxy Report were 0.87 and 0.91, respectively. Parent Proxy Report succeded more acceptable fit indices than Child Self Report. A statistically significant correlation was found between PedsQL-MFS and PedsQL-Neuromuscular Module (p<0.05). Moderate agreement was detected between parent and child.
Conclusion: The Turkish version of PedsQL-MFS was determined to be a reliable and valid tool to evaluate fatigue in 5-12 years old, ambulant children with DMD.
Duchenne Muscular Dystrophy (DMD) is the most common childhood neuromuscular disease which affects 1/3500–6000 male births.1,2 The disease is caused by a mutation in the dystrophin gene, which is localized at Xp21, and progressive muscle degeneration occurs over time.3,4 As seen in other neuromuscular diseases, the most prevalent symptoms are irreversible muscle weakness and fatigue, which quickly show themselves during daily activities.5 Fatigue is an important pathophysiological factor that hinders the realization of both physical and cognitive functions in individuals and causes limitations in exercise and activities. The levels of fatigue in daily living activities and functional skills are strongly correlated with the disorder.5,6 Fatigue decreases the physical capabilities and quality of life of individuals while increasing their dependency levels.7 It also creates a pathophysiological situation that affects the motivation of the individual to maintain their physical and cognitive functions, as seen in many different types of neuromuscular diseases.8
Fatigue is also an issue that may limit social participation. Patients with DMD who showed severe fatigue were found to have more problems with regard to physical functions, social functioning, mental health, physical pain status, and general health.9 Fatigue can be assessed using questionnaires that evaluate the loss of strength after exercise, changes in electromyographic activity recorded during an exercise period, and questionnaires that address fatigue directly and/or evaluate psychological aspects of fatigue.5 Special assessment scales for individuals with pediatric neuromuscular diseases—whose population mostly consists of DMD patients—are limited. However, experts need methods to determine the specific outcomes of promising drugs that have been developed in recent years. Also, considering the lack of a self-report assessment tool to assess fatigue in Turkish children with neuromuscular diseases; this study aimed to determine the psychometric features of the PedsQLTM-3.0 Multidimensional Fatigue Scale (PedsQL-MFS)-Turkish version in children with DMD.
Methods
This prospective, cross-sectional, observational study was performed in Hacettepe University, Faculty of Physical Therapy and Rehabilitation, Pediatric Neuromuscular Diseases Unit between January 2016-August 2018.
Subjects
A total of 71 patients with DMD, whose functional levels were between 1–3 (Level 1: The child is able to walk and climb the stairs, independently; Level 2: The child is able to walk and climb the stairs by using handrails, taking less than 12 seconds; Level 3: The child climbs the stairs slowly, taking more than 12 seconds) according to the Brooke Lower Extremity Functional Classification (BLEFC),10 aged between 5–12 years, who were on corticosteroids for more than 6 months, and who were still ambulant were included in the study with their parents. The children with severe physical and cognitive impairments that block performance and communication during the implementation of questionnaire and functional assessments, and the children with less cooperation were excluded from the study.
Hacettepe University’s Non-invasive Clinical Research Ethics Committee (No: GO 16/331) provided the ethical approval. Children (and their parents) who were directed to the Hacettepe University, Faculty of Physical Therapy and Rehabilitation, Pediatric Neuromuscular Diseases Unit for a routine physical examination after the diagnosis of DMD, signed a written consent form to be included in the study. This study followed the principles of Helsinki Declaration. All of the following assessments performed in this study were implemented approximately at the same time of the day for each child; frequently in the morning, rarely in the midday.
Method. PedsQ™-3.0 Multidimensional Fatigue Scale
The PedsQL-MFS allows to evaluate 2-18 years old pediatric patient population in terms of general symptomatic fatigue, and consists of 18 items. Test items are collected under three subgroups: general fatigue (six items, such as ‘feeling tired’, and ‘feeling too tired to do things that you like to do’), fatigue in sleep/rest (six items, such as ‘feeling tired at morning wake up’, and ‘needing lots of rest’), and cognitive fatigue (six items, such as ‘difficulty paying attention to something’ and ‘difficulty remembering what people tell you’). Clinical experience and studies on chronic pediatric diseases were considered to develop PedsQL-MFS.11,12 It contains questionnaires for the child to evaluate their own fatigue and for parents to evaluate their child’s fatigue. The child and adolescent questionnaires cover the ages 5–7, 8–12, and 13–18, while the young adult questionnaire covers the ages 18–25. For all child, adolescent, young adult, and parental questionnaires, scoring is done on a five-point Likert scale (0 = Never; 1 = Virtually Never; 2 = Sometimes; 3 = Frequently; 4 = Almost Always). The items are scored by linearly reversing the item scores (0=100; 1=75; 2=50; 3=25; 4=0) so that the higher the score, the better the quality of life, which indicates fewer symptoms of fatigue. Thus, a score of 0 indicates great fatigue, and a score of 100 points indicates less fatigue in PedsQL-MFS item scoring.11,13
The permission to perform the Turkish translation of the questionnaire was obtained from the developer of the source scale. The translation steps were as follows;
I. The English version of the questionnaire was translated into Turkish by 2 persons, independent of each other, that one of them with sufficient knowledge in the field of physiotherapy and rehabilitation.
II. Then the translations were compared and the differences were evaluated. A common opinion was reached and a single joint translation was created from 2 independent translations.
III. In the next stage, the Turkish questionnaire was translated back into English independently by 2 native speakers of English who also knew Turkish well, and did not know about the questionnaire.
IV. The new back-translated questionnaire was compared with the original questionnaire and the differences were evaluated.
V. After the translation process was over, all translations and reports were evaluated by a committee of experts consisting of methodologists, health experts, language experts and translators involved in the translation. Created questionnaires were corrected by this committee in terms of semantic, idiomatic, experiential, and conceptual equivalence.
VI. The final version of the questionnaire prepared by the committee was applied to 10 people and it was determined how the questionnaire worked during the application and the unidentified questions were corrected. Thus, the cultural adaptation of the Turkish version of the PedsQL-MFS questionnaire was completed.
Demographic properties including age (years), height (cm), weight (kg), and body mass indexes (BMI; kg/m2), as well as communication information, steroid use, and diagnostic information were recorded. The following functional evaluations were carried out.
The Evaluation of Functional Performance and Ambulation
Individuals participating in the study were subjected to timed performance tests, such as standing from supine position, 10 m walk, and ascending/descending four steps, which were considered to be a significant outcome measure to show functional performance in DMD.14 Another functional performance test—the six-minute walk test (6MWT), which has been used as a gold standard measurement for many clinical studies and which was found valid and reliable for patients with DMD—was also used.15 The distance walked in six minutes was recorded in meters.16
Individuals with DMD—depending on their progressive muscle weakness—tend to modify their movements in order to perform their daily living activities. The North Star Ambulatory Assessment (NSAA) grades daily activities that require ambulation as normal (2 points), modified (1 points), and dependent (0 points). The NSAA is a measure of 17 items that can be completed within 15 minutes, evaluating the skills necessary to maintain ambulatory function, from standing (item 1) to running (item 17). Each item in the NSAA is scored using a three-point scale. The total score is determined by summing the scores of all items, and ranges from 0 (no activity can be achieved) to 34 (all activities are achieved without help).17
Assessment of Quality of Life
The Turkish version of The Pediatric Quality of Life Inventory (PedsQL)3.0-Neuromuscular Module was used in the evaluation of the health-related quality of life of the patients. This scale was found to be valid and reliable in assessing the health-related quality of life of children with neuromuscular diseases between 2 and 18 years of age.18 The PedsQL-3.0 Neuromuscular Module was developed in the form of a parent report for children between the ages of 2 and 4, and in the form of both the child’s personal report and a parent report for children between 5 and 18 years of age. The scale consists of 25 items in 3 categories. Higher scores from the PedsQL-3.0 Neuromuscular Module indicate better health-related quality of life.18
Psychometric and Statistical Analysis
The IBM SPSS Statistics for Windows, Version 21 (Statistical Package for the Social Sciences, IBM Corp., Armonk, N.Y., USA) statistical analysis program was used to assess data obtained from the patients. Descriptive characteristics were identified as minimum, maximum, and mean± standard deviation (X±SD) for the quantitative data, while number (n) and percent (%) values were used for qualitative data. Data was analyzed in terms of normal distribution by using a histogram, the variation coefficient rate, the Skewness-Kurtosis, and Kolmogorov-Smirnov tests. It was determined that the data was not distributing, normally.
Feasibility
Each items’ missing values percentage was examined to determine the feasibility of the PedsQL-MFS Turkish version.19
Internal Consistency Reliability
The standardized Cronbach alpha coefficient was used to detect the internal consistency of the scale. For the Turkish version of PedsQL-MFS, the acceptable internal consistency was decided according to the criteria as Chronbach alpha coefficient >0.7.20
Test-Retest Reliability
The same physiotherapist applied the questionnaire twice to 71 children and their parents with a 2-week interval to test the test-retest reliability of the PedsQL-MFS. The test-retest reliability of the instrument was examined by calculating the intraclass correlation coefficient (ICC), and the ICC values between 0.1-0.3 were accepted as weak, 0.3-0.5 as moderate, and >0.5 as strong.21
Construct Validity
The factor structure of the Turkish version of PedsQL-MFS was investigated by confirmatory factor analysis (CFA). CFA determines if the new instrument presents similar factor solution with the original questionnaire. Thus, to perform analysis; root mean squared error of approximation (RMSEA), goodness-of-fit index (GFI), the chi-squared test (x2), comparative fit index (CFI), adjusted goodness-of-fit index (AGFI), and normed-fit index (NFI) were used. If the degrees of freedom (x2/df) is found <3.0, the GFI, CFI, NFI, and AGFI values are found more than 0.9, and RMSEA is determined <0.1; the instrument is accepted to behave similar with the original instrument.22 Also, a further analysis was performed to evaluate factor structure by using the exploratory factor analysis (EFA). Varimax rotation of the 18 items of the Turkish version of PedsQL-MFS was performed for principal component analysis.
Criterion-Related Validity
Correlations were examined between the scores (total and sub-scores) of the translated questionnaire and functional performance, ambulatory assessment, and quality of life assessment scores using Spearman’s correlation coefficient (rho) to test the validity of the questionnaire in Turkish patients with DMD.
Parent/Child Agreement
The Spearman’s correlation coefficient and ICCs were used for interpretation of agreement between the child’s self-report and parent’s proxy report. The significance levels according to the Spearman’s correlation coefficient were accepted as strong if r=0.7–1.00, moderate if r=0.30–0.70; and weak or insignificant if r=0.05–0.30. Levels were determined to be statistically significant at p < 0.05.
Results
Seventy-one boys with DMD aged 55 to 161 months, and 69 mothers (97.18%) and 2 fathers (2.82%) whose mean age was 37.05±3.75, and who were all graduated from high-school were included in this study. Demographic features of the children were given in Table 1. Sixty-two percent of the children were in level I, 31% in level II, and the others were in level III according to the BLEFC. The children were all on steroids and going to primary school.
Demographic characteristics of the patients (n=71).
Feasibility
Missing data from children’s self-reports and parents’ proxy reports were calculated in order to determine the feasibility of the Turkish version of the PedsQL-MFS. Missing data from the self-reports and parent proxy reports were 0.007% and 0%, respectively.
Internal Consistency Reliability
The internal consistencies of child self-report and parent proxy report determined by Cronbach’s alpha coefficient were 0.74 for general fatigue, 0.65 for sleep/rest fatigue, and 0.83 for cognitive fatigue, while 0.89, 0.84, and 0.91 for the parent proxy report, respectively.
Test-Retest Reliability
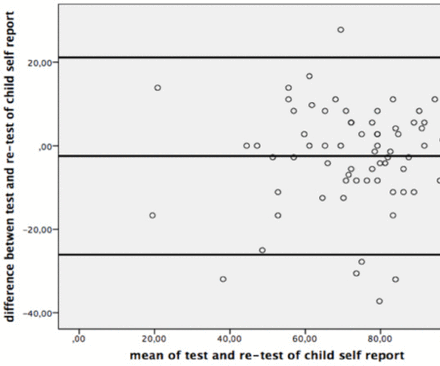
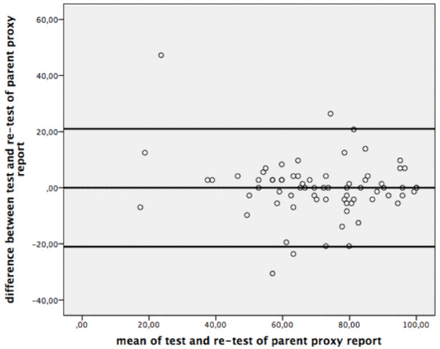
The ICC values of the PedsQL-MFS Turkish Version was shown in Table 2, and the Bland-Altman plots were given for the test-retest reliability of the questionnaire in Figure 1 and 2.
Test-retest reliability of the Turkish version of PedsQL Multidimensional Fatigue Scale.
Bland-Altman Plot for test-retest reliability of child self-report.
Bland-Altman Plot for test-retest reliability of parent proxy report.
Construct Validity
Table 3 presented the confirmatory factor analysis results of the 3-factor model of the child self-report and parent proxy report. The assessment tool had an acceptable RMSEA for the child self-report and an acceptable x2/df for child self and parent proxy reports. However, the RMSEA for parent proxy report and the CFI, GFI, and AGFI of the child self-report and parent proxy report were slightly below the cut-off value.
3-factor model results of confirmatory factor analysis.
The factor loadings of the items of the child self-report ranged within 0.34–0.75, 0.21–0.79, and 0.61–0.81 for the GF, SRF, and CF, while the factor loadings of the items of the parent proxy report ranged within 0.70–0.80, 0.74–0.87, and 0.58–0.83 for the GF, SRF, and CF, respectively. Therefore, parent proxy report of the PedsQL-MFS Turkish version was found to have more acceptable fit indices than the child-self report. However, the result of the estimated ranged correlation between the GF, SRF, and CF was 0.66–0.85. Three factors such as Factor 1 (cognitive fatigue), Factor 2 (general fatigue), and Factor3 (sleep/rest fatigue) for both the child self and the parent proxy reports were extracted by factor analysis with varimax rotation (EFA). The eigen values’ cut off was 1.0, with total variances of 51.7% and 68.1% for the self-report and proxy report, respectively (Table 4). Items one, four, five, and six split into the cognitive factor (Factor I), and items 11, 12, and 13 split into the general factor (Factor 2) in self-report which demonstrated highest load on a factor other than a priori hypothesized factor structure. All items from the parent proxy report agreed with the a priori hypothesized factor structure.
The Turkish Version of PedsQL Multidimensional Fatigue Scale Factor Loadings.
Criterion-related Validity
Table 5 demonstrated correlations between the PedsQL-Neuromuscular Module, the 10 m walk test, standing from supine position, ascending and descending 4 steps, the 6MWT, the NSAA, and the PedsQL-MFS total score and sub-scores. A statistically significant correlation was detected between the PedsQL-MFS and PedsQL-Neuromuscular Module scales (p < 0.05).
Correlation between the Turkish version of PedsQL-Multidimensional Fatigue Scale and other outcome measures.
Parent/Child Agreement
The ICCs between patients with DMD and their parents for PedsQL-MFS were as follows: total fatigue 0.62, general fatigue 0.42, sleep/rest fatigue 0.56, and cognitive fatigue 0.49. The ICCs were ranged in the moderate agreement. Correlations between DMD patients and their parents across the PedsQL-MFS were given in Table 6.
Inter-correlations between the child-self and parent proxy reports of Turkish version of PedsQL Multidimensional Fatigue Scale.
Discussion
This study provided the psychometric properties of the PedsQL-MFS Turkish version. It showed that the Turkish version of the PedsQL-MFS is a feasible, reliable, and valid instrument for evaluating fatigue in children with DMD between 5-12 years old. Furthermore, this study is the first study to evaluate multidimensional fatigue in DMD.
In 2013, Hu et al. found minimal missing data in their validity and reliability study of the Chinese version of the PedsQL Neuromuscular Module in 56 Chinese DMD children.23 Similarly, missing data was also minimal in our study. In addition, this study showed that both DMD children and their parents have good quality data on fatigue.
The intra-rater reliability was high in both the total and sub-scores of the PedsQL-MFS (Turkish version) parent proxy and child self-reports. This result supported the PedsQL-MFS as a reliable measure for children with DMD.
According to the CFA examination of the goodness of fit, the PedsQL-MFS (Turkish version) is structurally compatible with the original form. Moreover, the scale was found to be compatible with the fatigue of the population we studied. These results are similar to the CFA results for PedsQL-MFS translations into other languages.24,25 When EFA, which was used to examine the further construct validity of the PedsQL-MFS, was considered, the total variances were found to be higher in both children and caregivers than in previous studies, indicating that it gives more accurate results than other studies evaluating fatigue. However, the EFA results confirmed the exceptions regarding seven items in the child self-report. It is thought that this is caused by the limited number of children or the difficulty in understanding a subjective symptom such as fatigue.
PedsQL was determined to have a moderate-to-strong relationship between the PedsQL-MFS total and sub-scores and the PedsQL-Neuromuscular Module (excluding the PedsQL-Neuromuscular Module parent form and the PedsQL-Multidimensional Fatigue Scale child form general fatigue subscale). These results are similar to those of a study by Varni et al. performed in 200926 regarding the relationship between the Fatigue Scale and the PedsQL Generic Scale.
One of the most common problems that occurs secondary to muscle weakness is fatigue. It is known that this symptom starts with complaints such as lagging slightly behind peers in functional activities in early life, but it will also affect functions such as walking and climbing the stairs in the future, significantly affecting the child’s quality of life. Significant relationships between quality of life and fatigue overlap with studies in the literature related to the effects of fatigue on the quality of life of children with DMD. The reason for not having any relationship between PedsQL-MFS and ambulatory and performance tests that are frequently used in clinical practice may origin from the multidimensional aspect of the questionnaire which evaluates not only general fatigue caused by daily routine, but also sleep/resting fatigue and cognitive fatigue that are not directly related to the physical performance in daily activities. Thus, the result was considered to be acceptable when the structural characteristic of the questionnaire was regarded.
The moderate agreement between children with DMD and parent reports was found to be better than both adult and child studies of the PedsQL-MFS scale in literature.27,28 However, these results also showed that the data obtained from the children were not exactly same with the data obtained from the parents. Since the PedsQL-MFS has the ability to detect also the psychological aspect of fatigue with cognitive subheading, the perception of fatigue might be understood differently by children and parents, which prevented a “complete” or “exact” agreement between them. Thus, collection of data regarding fatigue from both parents and children is important in terms of accurate assessment of fatigue from different perspectives in clinical practice.
The limited number of patients with DMD and the inclusion of only physically active, ambulant children in current study can be considered as a limitation. Also, the missing information on the daily doses of steroids for each child is another limitation of the study.
In conclusion, the Turkish version of the PedsQL-MFS is a reliable and valid instrument to evaluate general, sleep/rest, and cognitive fatigue in 5-12 years old, ambulant children with DMD. The scale can be considered as a promising tool to be used as an outcome measure that evaluates fatigue and quality of life from the perspectives of parents and children with DMD in clinical trials in the future. Further studies are needed to be performed on a large number of patients with wide range of functionality to investigate broader use of the Turkish version of PedsQL-MFS.
Supplements
* Supplements will be considered for work including proceedings of conferences or subject matter covering an important topic
* Material can be in the form of original work or abstracts.
* Material in supplements will be for the purpose of teaching rather than research.
* The Guest Editor will ensure that the financial cost of production of the supplement is covered.
* Supplements will be distributed with the regular issue of the journal but further copies can be ordered upon request.
* Material will be made available on Saudi Medical Journal website
Acknowledgments
We would like to thank to the SCRIBENDI professional editing company (https://www.scribendi.com/) for English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received April 20, 2019.
- Accepted July 10, 2019.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.