Abstract
Objectives: To investigate the effects of various arm positions on median nerve conduction studies (NCSs).
Methods: This prospective cohort study, conducted at Adana City Training and Research Hospital between January and July 2023, included 20 healthy participants. Median NCSs were performed on the participants in three different standing positions with the elbow as the stimulation point: 1) with the arm adducted (P1), 2) with the arm anteflexed (P2), and 3) with the arm raised upwards (P3). We obtained median nerve compound muscle action potential (CMAP) latency, duration, peak-to-peak (PP) amplitude, onset-to-negative peak (OP) amplitude, negative area/duration. Three CMAPs were obtained in each position, and the mean and minimum/maximum values at each position were analyzed.
Results: The mean age (minimum–maximum) of the participants (11 male, 9 female) was 28.5±6.5 (20–42) years. Median nerve CMAP means (median) of latency/negative duration in the P1, P2, and P3 positions were 6.82±0.59 (6.83)/5.50±0.76 (5.39) ms, 6.99±0.56 (7.02)/5.72±0.73 (5.73) ms, and 7.03±0.58 (7.12)/5.79±0.80 (5.83) ms. Median nerve CMAP mean/minimum latency and negative duration were lowest in the P1 position (p<0.05). The mean median nerve CMAP OP amplitude was highest in P3 than P1 or P2 (p=0.042 and p=0.048).
Conclusion: Median NCS results differed based on the position of the arm.
Nerve conduction studies (NCSs) provide unique information on the physiology of the peripheral nervous system. Myelinated sensory and motor nerves can thus be examined through routine NCS, allowing the diagnosis of diseases involving the peripheral nervous system, such as mononeuropathy, plexopathy, or polyneuropathy.1,2 Many factors, such as skin temperature, age, and height, can affect NCS findings.1,2 Limb position can also affect NCS, as evidenced by the slowing of ulnar motor nerve conduction velocity (NCV) when the elbow is extended compared to when it is flexed.3,4 Another example is that peroneal motor NCV is faster when the hip is flexed than when it is extended.5 The NCS differences among various extremity positions are reported to be related to nerve stretching and changes in muscle length.1,3,5,6 The median nerve is frequently evaluated in routine NCS, and median NCSs play a key role in diseases such as carpal tunnel syndrome or brachial plexopathy.7,8 Therefore, it is important to know which conditions affect median NCSs. The length and physiological characteristics of the median nerve are known to be affected by arm and wrist positions; however, these studies performed median NCSs in the wrist positions and evaluated median nerve anatomy in the arm positions.9-11 In this study, therefore, we aimed to investigate whether there is a relationship between median NCS and various extremity positions.
Methods
This prospective cohort study was conducted at the Clinical Neurophysiology Laboratory at the Adana City Training and Research Hospital (ACTRH) between January and July 2023. Lesions or degeneration in part or the entirety of the median nerve can lead to abnormalities in median NCSs performed in different arm positions. Therefore, individuals with chronic diseases, such as mononeuropathy, polyneuropathy, or paresthesia; weakness in their extremities; nerve conduction study findings consistent with Martin-Gruber anastomosis; or abnormal neurological examinations were excluded from this study. The protocol for this study was approved by the ACTRH Clinical Research Ethics Committee (no.: 118/2304, date: 12/15/2022). The procedures applied to all human participants were in accordance with the ethical standards of the Helsinki declaration and the Clinical Research Ethics Committee of ACTRH. The procedures applied to the participants in this current study were explained in detail, and the participants provided written informed consent that they approved the procedures.
Neurophysiological testing
The NCSs were performed on all participants using a Cadwell Sierra Summit electromyography unit (Cadwell, Kennewick, WA, USA). Median NCSs were performed as previously described, using the right upper extremity.12 Care was taken to ensure that the extremity temperature was above 32°C. If it was not, a hair dryer was used to bring the arm up to temperature. The high- and low-pass filters for the motor and sensory NCSs were 20 Hz–10 kHz and 20 Hz–2 kHz. The sensitivities for motor and sensory NCSs were 2–5 mV/division and 10 μV/division. The sweep speed was set at 5 ms/division for the motor NCS and 1 ms/division for the sensory NCS. Surface electrodes were used for both nerve stimulation and recording. Round silver electrodes 10 mm in diameter were used as the active and reference electrodes. The stimulation was done supramaximally, and the duration of stimulation was 0.1-0.2 ms. Routine median NCSs were performed with the patient in the sitting position. The median nerve compound muscle action potential (CMAP) was recorded from the abductor pollicis brevis (APB) muscle as follows: active electrode was placed on the belly of the APB muscle and reference electrode was placed on the APB tendon, with the reference electrode 3–4 cm distal to the active electrode. Median motor nerve stimulation points were the wrist and elbow area. The median nerve stimulation point on the wrist was 5 cm proximal to the recording electrode. The median nerve CMAP amplitude was calculated using both peak-to-peak (PP) and onset-to-negative peak (OP) methods. To exclude Martin-Gruber anastomosis, the changes in the median and ulnar nerve CMAP amplitudes during proximal and wrist stimulation was examined, as was whether the median CMAP was obtained with the stimulation of the ulnar motor nerve and/or vice versa. The median sensory NCS was applied antidromically. The distance between the active electrode placed on the second finger and the stimulation point on the wrist was 14 cm, to achieve the median compound nerve action potential (CNAP). The amplitude of the median nerve CNAP was determined using the PP method. The median sensory NCV was calculated using the peak latency. Among the ten median nerve F-waves obtained, the F-wave with the shortest latency was selected.
Procedure
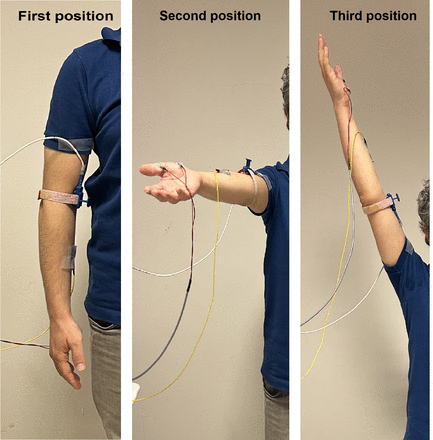
Median NCSs were performed with the participants in a standing position. The stimulator was fixed tightly with a band in the elbow area to prevent the stimulation point from changing with arm movement, and the CMAP of the median nerve was recorded from the APB muscle. The active and reference electrodes were placed on the ABP muscle and tendon as previously described. The electrodes were placed tightly with adhesive tape to prevent their locations from changing with arm movement. The electrodiagnostic testing procedure was performed in three parts: 1) in the first position (P1), participants’ arms were hanging down; 2) in the second position (P2), participants flexed their shoulders and extended their arms forward for 90° between the arm and body; and 3) in the third position (P3), participants raised their arms for approximately 180° of shoulder flexion (Figure 1). The arm position angles were obtained using a goniometer. In all positions, the participants’ elbows were extended, and the wrists were neutral. The participants were asked to keep their fingers and hand muscles relaxed for all testing. Three CMAPs were achieved to avoid variability in NCS results. The CMAPs were obtained 10 s apart at each position with a 1-min interval between positions. The latency, amplitude, negative duration, and negative area of the median nerve CMAP were included in our analyses. The CMAP latency, amplitude, negative duration, and negative area values obtained at each position were divided into three, and mean values were obtained. Additionally, the minimum latency, negative duration, maximum amplitude, and negative area were measured for all three CMAPs.
- Arm positions in participants.
Statistical analysis
Median, mean±standard deviation (SD), and min-max were used to define numerical data. Categorical variables were expressed as frequency and percentage. The Friedman test was used to compare numerical data among the three dependent groups, while the Wilcoxon test was used to compare numerical data between the 2 dependent groups. Bonferroni correction was utilized for post-hoc analyses. The sample size was calculated using the G*Power Version 3.1.9.6 program (Düsseldorf, Germany). Studies related to peripheral nerves and the various arm positions have been reviewed.3,4 The sample size was calculated with Wilcoxon signed-rank test, which provided a minimum number of participants required for the study of 16, with a standard type 1 error rate (0.05) and power of 0.80.3,4 Therefore, 20 healthy individuals were included in this study. Statistical significance was set at p<0.05 and SPSS v.22.0 (SPSS IBM Corp., Armonk, NY, USA) was used to perform the statistical analyses.
Results
A total of 20 participants were included: 11 males (55%) and 9 females (45%). The mean age of the participants was 28.5±6.5 (20-42) years and mean of their body mass index (min-max) was 24.7±3.5 (19.9-32.8) kg/m2. From the routine median NCSs, the mean (min–max) median nerve CMAP PP amplitude was 14.9±4.6 (7.3–23.3) mV, mean OP amplitude was 8.9±2.8 (4.1–15.6) mV, mean distal latency was 2.9±0.3 (2.5–3.7) ms, mean motor NCV across the wrist-elbow segment was 58.8±4.7 (52.7–70.1) m/s, mean median nerve F-wave minimum latency was 27.6±2.3 (23.4–32.8) ms, mean median CNAP amplitude across the second finger-wrist segment 72.8±23.8 (32.9–128.3) μV, and median sensory NCV was 45.7±3.5 (41.7–54) m/s.
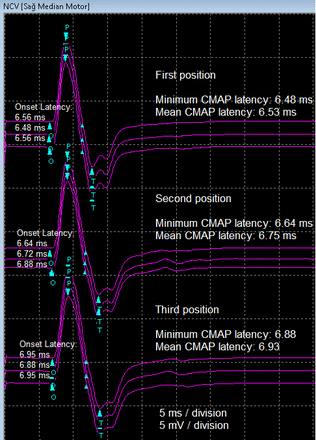
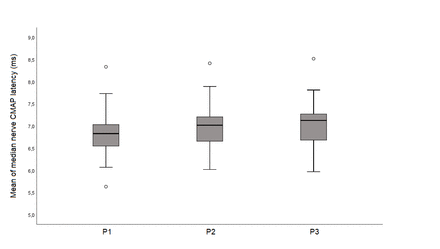
The median NCS findings obtained in the P1, P2, and P3 positions, as well as a comparison of the findings among the positions are shown in Table 1. Comparisons of the median nerve CMAP values between the paired groups are presented in Table 2. The median nerve CMAP findings of a participant obtained in different arm positions are shown in Figure 2. Figure 3 illustrates the comparison of CMAP distal latencies at the P1, P2, and P3 positions.
- The comparison of median NCS findings between the arm positions.
- Pairwise comparisons of median NCS findings.
- The median nerve CMAP findings of a participant were obtained in different arm positions. CMAP- compound muscle action muscle.
- The comparison of CMAP distal latencies in different arm positions. CMAP- compound muscle action muscle.
Discussion
In this study, we investigated whether there was an association between median NCS findings and extremity position. We found that when the participant was standing and the arm was in the anatomical (neutral) position, median nerve CMAP latency occurred earlier than when the arm was raised. This indicates that the median motor NCV across the forearm and wrist segments is faster when standing with the arm adjacent to the trunk than when the arm is raised.
It is well documented that extremity positions affect NCSs.1–6 Across the elbow segment, the ulnar NCV is faster when the elbow is flexed than when the elbow is extended.3,4 Studies have reported that median NCV is affected by the position of the wrist,9 and peroneal motor NCV is affected by the position of the knee.5 Differences in latency among various extremity positions found in this study may be related to nerve stretching and/or changes in nerve length as a result of extremity posture.1,3,5 Previous studies have reported that the length of the median nerve increased with external shoulder rotation or abduction and elbow-wrist extension.10,11 In this study, therefore, the median nerve may have increased in length when the participants raised their arms. If this was the case, we would expect the median nerve CMAP latencies to occur earlier in this position than others. Additionally, the distance at which nerve conduction progressed should have been shortened, in turn reducing temporal dispersion. Therefore, a reduction in the median nerve CMAP-negative duration was expected; however, the opposite results were obtained in this study. This can be explained by the stretching of the median nerve across the axillary segment, as the median NCV was faster in the axillary region or arm segment than in the forearm segment. A previous study has reported, however, that tension in the forearm increases with arm-shoulder abduction, wrist extension, and elbow extension.10 Another reason for this unexpected outcome in our study may be related to the testing method utilized. The arm positions evaluated in this study were the anatomical (neutral) position, anteflexion, and extension. Although a previous study reported that the median nerve lengthens when the arm is extended, the effect of anteflexion is unknown.11
The median nerve may be compressed in the axillary region during extension, which may explain the delayed occurrence of CMAP latency when the arm is raised. Additionally, deterioration in the blood flow of the nerve due to stretching may also occur.10,13 It may be difficult to claim, however, that such a short-term postural change can result in physiological changes in the nerve.13 Nerve stretching due to extremity position may not affect the NCS alone. It is important to remember that muscle length can also change with arm movement;1,5 therefore, in this case, both the active and reference electrode locations may migrate. All of these factors can affect the CMAP amplitude and duration.
The median nerve CMAP amplitude and negative area were higher when the arm was anteflexed and raised than in the neutral position, which may be explained by the median nerve becoming more tense and superficial with flexion. In this position, it is easier to stimulate the median nerve, in turn increasing median nerve CMAP amplitude. Another reason for these findings may, therefore, be the aforementioned change in the muscle.
Gravity may also be one of the reasons why the median NCV across the forearm segment is faster in the P1 than the P3 position,14,15 as a prior study reported that as gravity changes, the latencies of the H-reflex and M-wave also change.14 We found in this study that when the participants were standing in the P1 position, the recording electrodes were closer to the ground and the direction of nerve conduction was the same as that of gravity. When the arms were raised, however, the recording electrodes were farther away from the ground than in the neutral position, and the direction of nerve conduction was opposite to that of gravity. Moreover, a previous study reported that H-reflex duration decreases as gravity increases,14 consistent with the finding that median nerve CMAP durations are lower in the P1 than the P3 position. Studies have also reported that there may be a relationship between current velocity and gravity. As gravity decreases, the current may slow down, potentially causing changes in the operation of the devices.16 In this case, it is conceivable that in environments with lower gravity than Earth, the NCV may slow down relative to that on Earth, as the nerve conduction moves in the direction of gravity. Further studies are needed, however, to prove that gravity affects NCS. Gravity can also cause changes in electroencephalography;17 therefore, it can affect both nerve conduction and the potential produced by the nerves.
This study had some limitations. Changes in muscle length and active/reference electrode location due to arm movements may have caused the CMAP changes, as limb movements may affect the proximity of the active electrode to the muscle endplate zone, resulting in changes in CMAP amplitude, latency, and/or motor NCV.18 It should be noted, however, that in this study, the electrodes were tightly taped to the patient to minimize migration. Additionally, this study was conducted with the participants standing, which can also be considered a limitation; therefore, studies conducted with the participant in the supine position may be beneficial to verify or disprove our results. Furthermore, studies involving a mechanism such as a tilt table to achieve positions that are not in the same direction as gravity may be of interest. In such cases there should also be minimal-to-no movement of the stimulator or electrodes. We did not include participants’ height or body mass index in our analyses, which could also be a limitation of this study. Future studies on limb position and NCS which evaluate these individual characteristics, therefore, may provide a better understanding of the physiology of nerve conduction and the factors affecting NCS. Moreover, investigating the relationship between limb position and NCS in patients with diseases that cause neuropathy, such as diabetes mellitus, by comparing them with healthy individuals may enhance the understanding of the pathophysiology of neuropathy.
In conclusion, we found that the median nerve CMAP latency, amplitude, negative duration, and negative area across the wrist and forearm segments differed among different arm positions while the participant was standing. These findings may be related, therefore, to changes in the length of the median nerve and APB muscle due to arm position and/or gravity.
Acknowledgement
We would like to thank “Editage” (https://www.editage.com/) for English editing.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received April 7, 2024.
- Accepted August 27, 2024.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.