Abstract
Objectives: To determine the effects of all-trans-retinoic acid (ATRA) on the post-stroke inflammatory response and elucidate the underlying molecular mechanisms.
Methods: This animal experiment was conducted at Central Laboratory, the First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China during 2020-2022. Sprague-Dawley rats were subjected to middle cerebral artery occlusion (MCAO) for 1.5 h, and treated with ATRA at 2 and 24 h after reperfusion. Neurological deficit scores on behavioral tests, and cerebral infarct volume, microglial polarization, and the expression levels of inflammatory cytokines and proteins associated with TLR4/NF-κB signaling were assessed.
Results: The ATRA administration reduced cerebral infarct volume and ameliorated neurological deficit scores in MCAO rats. Additionally, ATRA relieved cerebral edema and downregulated the secretion of proinflammatory cytokines after stroke. Finally, ATRA attenuated the polarization of the microglia toward the M1 phenotype and promoted the activation of the beneficial M2 phenotype; the underlying mechanism potentially involved the suppression of the TLR4/NF-κB signaling pathway.
Conclusion: The ATRA treatment promoted functional recovery in an experimental model of ischemic stroke by attenuating neural inflammation. ATRA potentially modulated microglia-mediated neuroinflammation via the downregulation of the TLR4/NF-κB signaling pathway, which makes it a candidate treatment for post-stroke neuroinflammation.
Globally, stroke is the leading cause of disability and the second leading cause of death.1,2 Ischemic stroke (IS) accounts for the vast majority of all cases of stroke.3 Currently, intravenous thrombolysis and endovascular thrombectomy are the 2 most effective treatments available for IS; however, these treatments must be administered within a narrow time window, so only a small number of patients are able to receive them.4,5 A growing body of research indicates that inflammation after stroke may greatly affect patient outcomes, even among those who successfully undergo vascular recanalization. Therefore, the inhibition of the post-stroke inflammatory response is a novel therapeutic strategy for IS.6,7
The mechanism of inflammation after stroke is complex, ambiguous, and involves multiple types of inflammatory cells and inflammatory factors. Recent studies have reported that microglia are crucially involved in the post-stroke inflammatory response, and intriguingly, exert both beneficial and detrimental effects during IS.8-10 Microglia can be divided into 2 types based on their phenotypic characteristics: M1 microglia, which secrete pro-inflammatory factors and aggravate tissue damage, and M2 microglia, which secrete anti-inflammatory factors, suppress the inflammatory response, and promote tissue repair.11 Thus, regulating microglial transformation to the M2 phenotype after IS is a potential therapeutic target for the inhibition of post-stroke inflammation.
Recent studies have found that the toll-like receptor 4 (TLR4) and nuclear factor-kappa B (NF-κB) signaling pathway is closely related to ischemic injury in various organs, and is also involved in mediating the post-IS inflammatory response.10,12 Moreover, studies in the rodent central nervous system have shown that TLR4 is mainly expressed in the microglia,13 which suggests that TLR4 is a key signaling protein for microglial regulation. In the event of an IS, TLR4 recognizes the damage-associated molecular pattern (DAMP) and triggers its downstream adaptor protein myeloid differentiation factor 88 (Myd88), which then activates the nuclear transcription of NF-κB, causing the release of numerous pro-inflammatory factors such as interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α), and thereby exacerbating the inflammatory response.14,15
All-trans retinoic acid (ATRA) has been successfully applied as a differentiation inducer during tumor treatment in clinical practice. Recent reports have shown that ATRA can protect against inflammation in important organs such as the heart, brain, and kidneys.16-19 Our previous study revealed that ATRA downregulated the levels of inflammatory factors and upregulated the levels of anti-inflammatory factors in plaques in a rabbit carotid atheroma model, and thereby inhibited the inflammatory response and improved plaque stability. However, the specific mechanism underlying these changes was unclear.
The aim of the present study is to determine the effects of ATRA on the post-IS inflammatory response and elucidate the mechanism of inflammatory regulation in a rat model of middle cerebral artery occlusion (MCAO). We hope that our findings will facilitate the discovery of new therapeutic targets in IS and provide an experimental basis for the development of novel drugs.
Methods
This animal experiment was conducted at Central Laboratory, the First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China during 2020-2022. This research study was conducted in accordance with the tenets of the Declaration of Helsinki, and the “Principles for Ethical Review of Drug Clinical Trials20” were followed. The animal experimental protocols were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University, and the Guide for the Care and Use of Laboratory Animals and Stroke Treatment was followed.
A total of 45 adult male Sprague-Dawley (SD) rats (250–280 g) were obtained from the Animal Experiment Center of Southern Medical University (Guangzhou, China) and were adaptively housed for 1 week in the First Affiliated Hospital of Guangdong Pharmaceutical University (Guangzhou, China). The animals were housed under a 12:12 h light:dark cycle, to simulate a natural environment. Food and water were freely accessible. The animals were randomly divided into 3 groups (n=15/group) by using a randomized block design: sham group, MCAO group, and ATRA group.
We established a rat model of IS by inducing MCAO with the filament occlusion method to build upIS model. The rats were anesthetized with 4% sodium pentobarbital (40 mg/kg body weight, i.p.). A surgical filament was inserted into the common carotid artery, passed into the internal carotid artery, and further advanced until it reached the middle cerebral artery (MCA). The filament was kept in place in the MCA for 1.5 h. During this procedure, the rats’ body temperature was maintained at 37°C±0.5°C by using a heating light. The animals in the sham group were subjected to the same procedure, except that no filament was inserted.
ATRA (R2625, Sigma, St. Louis, MO, USA) was administered (10 mg/kg, i.p.) as a bolus dose at 2 h and 24 h after reperfusion following MCAO to the rats in the ATRA group. The animals in the sham and MCAO groups received equivalent volumes of PBS (i.p.). The drug administration was performed in a double-blinded manner, with both the experimenter and the assessor being unaware of the experimental groups.
Neurological deficit scores were calculated at 2, 24, 48, and 72 h after ischemia-reperfusion. The neurological deficit scores were measured using the Zea-Longa method, on a 0–4-point scale: 0 points, no significant neurological deficit; 1 point, adduction or flexion of the affected forelimb occurs during tail lifting; 2 points, turns in a circle to the paralyzed side when crawling; 3 points, body imbalance with the trunk inclined to the ipsilateral side; and 4 points, unable to walk or unconscious.21
The cerebral infarct volume was measured using staining with 2,3,5-triphenyltetrazolium chloride (TTC; T8877, Sigma, St. Louis, MO, USA). The brain tissues of the experimental rats were cut into six 2-mm-thin coronal slices in the MCA territory, stained with a 3% TTC solution, and fixed with a 4% paraformaldehyde solution in PBS. The cerebral-infarct volume was determined using ImageJ software, which calculated the ratio of the area of the unstained region (infarct) to that of the positively stained region (healthy brain tissue). The infarct volume ratio was calculated as the ratio of the sum of the infarct area in each brain slice to the sum of the total area of each brain slice.
Anesthetized rats were subjected to cardiac perfusion with 0.9% normal saline followed by fixation with 4% paraformaldehyde. Paraffin sections were made from coronal slices taken 0.5 mm behind the optic chiasm. The slices were then cut into 0.5-μm-thin sections and mounted on slides. The slides were placed in a pure xylene solution for 10 min to completely dissolve the paraffin wax; the dewaxing step was performed twice in total. Following dehydration, the slices were successively placed into 75%, 50%, and 25% xylene ethanol solution for 10 min each, and then successively placed in 100%, 95%, 90%, 80%, 70%, 60%, and 50% ethanol solution for 10 min each. Finally, the slides were stained with hematoxylin and eosin for morphological observation.
Blood samples were collected from the abdominal aorta immediately after the animals were sacrificed, and allowed to stand at 4°C for 2 h. The samples were centrifuged at 4°C and 3,000 rpm for 20 min, and the resultant serum was subjected to enzyme-linked immunosorbent assay (ELISA; Meimian, Jiangsu, China), to measure the IL-6 level. The ELISA was performed in strict accordance with the manufacturer’s instructions. In brief, 50 μL of standard product at different concentrations (160, 80, 40, 20, 10, and 0 pg/mL) was added to the standard product wells, while 40 μL of the sample diluent and 10 μL of the test sample were added to the test wells. All the wells were coated with 100 μL capture antibody at 4°C, and washed thrice with the washing liquid. Then, 200 μL assay diluent was used to block irrelevant proteins at 37°C for 1 h. Following 5 washes with the diluent solution, the wells were patted dry. The color developing agent was added to each well and incubated for 20 min at room temperature. Finally, the absorbance at 450 nm was quantified using an ELISA reader, relative to the IL-6 protein standards.
Immunofluorescence analysis was applied to evaluate the activation and phenotypes of microglia. Paraffin-embedded sections of brain tissues were subjected to microwave heat-induced epitope retrieval. The specimens were then washed with PBS and incubated at 4°C for 18 h with mouse anti-rat Iba-1 antibody and CD206 antibody (1:500; Abcam, Cambridge, MA, USA). The specimens were again washed with PBS and further incubated for 2 h with goat serum anti-mouse IgG Alexa Fluor 488 (1:200; Cambridge, MA, USA). The cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). After being washed thrice with PBS, the specimens were mounted in Prolong Gold Antifade reagent, and imaged using a confocal fluorescence microscope.
Sensorimotor cortical tissues of the rats were harvested and homogenized in radioimmunoprecipitation assay buffer and nuclear fractionation buffer. The tissues were centrifuged at 4°C and 3,000 rpm for 30 min, and the resultant supernatants were used to measure the total protein concentration as well as the concentrations of cytosolic proteins and nuclear proteins. All protein concentrations were measured using the bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA), and the manufacturer’s instructions were followed. Next, 50-μg aliquots of the proteins were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene fluoride membranes (Merck Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat dry milk for 2 h at room temperature and then incubated overnight at 4°C with the following primary antibodies: anti-TLR4 (1:500) or anti-NF-κB p65 (1:500) or anti-Myd88 (1:1000, Affinity Biosciences, Jiangsu, China), anti-IL-6, and anti-β-tubulin (1:1000, Affinity Biosciences, Jiangsu, China). This was followed by incubation with a horseradish peroxidase-conjugated antibody (1:5000) at 4°C for 60 min. Protein bands were identified using an enhanced chemiluminescence kit and the Bio-Image Analysis System. The optical density was calculated, and the expression levels of the proteins were presented as the densitometric ratio of the proteins to anti-β-tubulin.
Statistical package for the social sciences 26.0 software was used for statistical analysis of the data, and Graphad Prism 8.0.2 was used for mapping± SEM) was used to represent the results. T-test was used between two independent samples. One-way analysis of variance was used for multi-group data analysis Tukey’s method was used for pairwise comparison, and p-value<0.05 was considered statistically significant.
Results
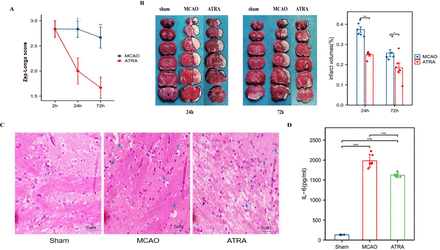
As described previously, the rats in the ATRA group were administered ATRA (10 mg/kg, i.p.) while those in the sham and MCAO groups were administered the same volume of PBS at 2 h and 24 h after reperfusion. Compared with the sham and MCAO groups, the rats in the ATRA group showed significantly lower neurological function scores and significantly smaller cerebral infarct volumes (p<0.01; Figure 1a, 1b). Li et al22 have reported that the cerebral infarct volume peaks at 24 h in the MCAO model, and our results were consistent with this. We found that treatment with ATRA significantly decreased the infarct volume at 24 h and 72 h after focal ischemia, as compared with the MCAO group (p<0.01), and the decrease was more pronounced at 72 h than at 24 h (Figure 1b).
- The ATRA treatment protected against acute ischemic stroke. The SD rats were treated with ATRA (10 mg/kg, i.p.) or an equal volume of PBS at 2 h and 24 h after reperfusion following 90 min of cerebral ischemia. The rats were sacrificed at 24 h and 72 h after MCAO. a) Neurological deficit scores at 24 h and 72 h after MCAO. The values shown are mean±SEM (n = 6 per group). *p≤0.01 (one-way ANOVA). b) Infarct volume was quantified using TTC (red)-stained coronal sections. The values shown are mean±SEM (n=6 per group). *p≤0.01 (t-test). c) Vacuoles (blue arrows) are seen on hematoxylin and eosin-stained brain tissue. (d) IL-6 expression levels in the 3 groups. The values shown are mean±SEM (n=6 per group). *p≤0.05 (t-test), **p<0.01 (t-test).
To understand the relationship between the changes in the brain structure and function of the rats, we used hematoxylin and eosin staining to microscopically examine the pathophysiological changes in the brain tissue. We found that the brain cells of rats reperfused after ischemia showed edema, vacuolization, and nuclear contraction changes. The number of abnormal cells in the adjacent basal ganglion region was lower in the ATRA group than in the MCAO group (Figure 1c).
To determine whether ATRA inhibits the inflammatory response after stroke, we measured the serum level of the classic pro-inflammatory factor IL-6. We found that the IL-6 concentration in the sham group was at the baseline level, while it was significantly increased in both the MCAO and ATRA groups (p<0.01). Interestingly, the serum level of IL-6 was significantly lower in the ATRA group than in the MCAO group (p<0.01), indicating that ATRA had an inhibitory effect on systemic inflammation after stroke (Figure 1d).
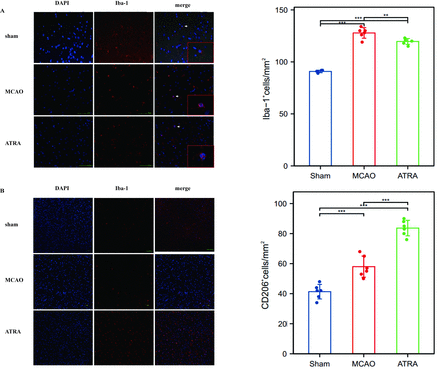
As the above results suggested that ATRA inhibits the inflammatory response, we explored whether ATRA regulates the post-stroke inflammatory response via the microglia. To examine the influence of ATRA on microglial activation, we performed immunofluorescence staining for Iba-1 (a marker of M1 microglia) and CD206 (a surface marker of M2 microglia). The results showed that in the sham group, the microglia were in a resting state, appeared highly branched, and exhibited multiple synapses. In contrast, in the MCAO and ATRA groups, the Iba1-positive microglia were in an activated state, and their cell morphology was round or rod-shaped, with some cells showing an “amoeboid” appearance (Figure 2a). Some differentiated synapses were observed in the ATRA group. Additionally, the expression of CD206-positive microglia (i.e., the M2 phenotype) was low in both the MCAO and sham groups, and high in the ATRA group (Figure 2b).
- Microglial Iba1 and CD206 expression in the study groups. (a) Immunofluorescence detection of Iba-1 (red); the nuclei are stained with DAPI (blue; 400× magnification). (b) Immunofluorescence detection of CD206 (red); the nuclei are stained with DAPI (blue; 100× magnification). Quantitative analysis of the Iba-1-positive microglia and CD206-positive microglia is also shown (n=6 in the sham group, and n=6 in the MCAO and ATRA groups). The values shown are mean±SEM. ns=not significant. *p≤0.05 (t-test), **p<0.01 (t-test).
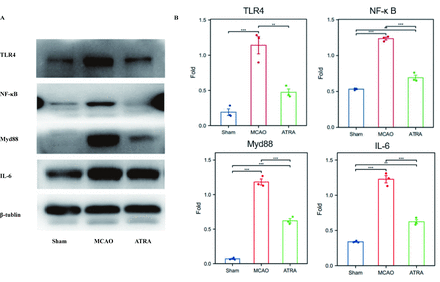
TLR4 is closely related to microglial regulation and mediates NF-κB signaling, which is involved in the regulation of multiple inflammatory responses. Hence, we further explored whether ATRA regulates the post-stroke inflammatory response by modulating the TLR4/NF-κB signaling pathway. We analyzed the expression levels of proteins associated with TLR4/NF-κB signaling by performing western blotting. We found that compared with the sham group, the MCAO group showed significantly increased expressions of TLR4, p65 NF-κB, Myd88, and IL-6. In addition, the protein expressions of TLR4, p65 NF-κB, Myd88, and IL-6 significantly decreased after ATRA treatment (Figure 3).
- Effects of ATRA on TLR4 and NF-κB signaling in ischemic stroke. a) Representative bands of TLR-4 and NF-κB by western blot assay based on an internal control of β-tubulin. b) Quantification of the western blot results (n=6 per group). *p<0.05 (t -test), **p<0.01 (t- test).
Discussion
The regulation of the inflammatory response after stroke is a research hot spot and a challenging issue in the treatment of ischemic stroke.23 The ATRA has long been used for the treatment of acute myeloid leukemia; however, an increasing body of research indicates that it may be a novel strategy to regulate.24-26 The ATRA has been shown to exert anti-inflammatory and plaque-stabilizing effects in rabbit atherosclerotic models.27 Our present study demonstrated that ATRA can regulate neural inflammation after stroke by regulating the polarization and function of the microglia. The underlying molecular mechanism may involve the TLR4/NF-κB signaling pathway.
The current study revealed that ATRA exerts neuroprotective as well as immunomodulatory effects, which are mediated through the regulation of microglia in the brain tissue after acute ischemic stroke. Previous studies have suggested that ATRA may protect against cerebral ischemic injury in rats with MCAO.26,28 Consistent with this, our study demonstrated that ATRA can significantly decrease the cerebral infarct volume and ameliorate the neurological deficit resulting from ischemic stroke. Inflammatory cytokines, such as IL-6, have been reported to be upregulated immediately following stroke.29,30 The present study confirmed that ATRA significantly reduced the serum IL-6 level following MCAO. These results indicate that the neuroprotective effects of ATRA are closely linked to its anti-inflammatory properties. However, we did not measure the IL-6 level in the brain tissue, and the direct effect of ATRA on cerebral IL-6 expression needs to be investigated in future studies.
Microglia are the first immune cells to reach the lesion area after a stroke,31-33 thus, regulating the activation and function of microglia is a crucial therapeutic target for ameliorating the post-stroke neural inflammatory response. However, post-stroke neuroinflammation involves a complex cascade of signals and a variety of cells, and it is unclear which link plays a major role. Studies have shown that microglial activation is a double-edged sword. This is because activated microglia can differentiate into the classical M1 phenotype, which produces reactive oxygen intermediates and secretes pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α, and thereby mediates inflammatory tissue damage.31,34 Alternatively, activated microglia can polarize into the M2 phenotype and secrete anti-inflammatory cytokines such as IL-10, IL-4, and transforming growth factor (TGF)-β, which produce neuroprotective effects by suppressing inflammation and promoting tissue repair, remodeling, and vasculogenesis.33,35,36 Therefore, inhibiting the activation of M1 microglia or promoting the transformation of M1 microglia to the M2 phenotype has neuroprotective effects.37 Our study showed that ATRA promotes the transformation of M1 microglia to the beneficial M2 phenotype or helps to activate M2 microglia.
Although microglial polarization is strongly associated with neuroinflammation, the specific mechanism underlying the regulation of microglial polarization is not clear. The TLR4 is widely expressed in microglia, and TLR4-mediated inflammatory signaling pathways may be key targets for microglial regulation. The TLR4 is a membrane receptor that recognizes damage-associated molecular patterns (DAMPs); when activated, TLR4 triggers NF-κB signaling and induces the production of proinflammatory cytokines such as IL-1β and IL-6.34,38-41 Therefore, we hypothesized that inhibiting TLR4/NF-κB signaling may promote the polarization of the microglia towards the favorable M2 phenotype and reduce the release of inflammatory factors. Our findings indicated that ATRA could downregulate TLR4/NF-κB signaling. Thus, the neuroprotective effects of ATRA may be mediated through the regulation of microglial polarization by downregulating the TLR4/NF-κB signaling pathway and thereby reducing post-stroke inflammation.
Our findings provide important research data for exploring the mechanism of inflammation regulation in ischemic stroke; however, the limitations of our study should be acknowledged. First, we did not compare drug concentration gradients. Second, the inflammatory mediators measured in this experiment were limited. Finally, this research lacks cellular studies of drugs and TLR4/NT-κB pathway blockade. Therefore, further experiments to provide direct evidence are needed.
Conclusion
Our animal experimental study revealed that the anti-inflammatory effects of ATRA after cerebral ischemia induced by MCAO are potentially mediated via the downregulation of TLR4/NF-κB signaling to regulate microglial polarization to the beneficial M2 phenotype and impede the expression of proinflammatory cytokines. Therefore, methods to promote the differentiation of microglia cells into the favorable M2 phenotype merit more attention. The ATRA is currently used to treat acute promyelocytic leukemia, yet it has not yet been applied in clinical practice for regulating the inflammatory response in disease. ATRA has been shown in several studies to have a protective effect against inflammatory responses in ischemic stroke; it can regulate neutrophils, reduce the formation of neutrophil extracellular traps, and improve the permeability of the blood-brain barrier.25,26 Considering its neuroprotective potency in stroke, we believe that further experimental and clinical research into the ATRA treatment of stroke are warranted.
Acknowledgement
We would like to thank thank Sofia Fields Author Services (https://sofiafields.com) for English language editing.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received November 30, 2023.
- Accepted June 14, 2024.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.