Abstract
Objectives: To utilize our tertiary center’s experience with Temporal lobe epilepsy (TLE) and Temporal plus epilepsy (TPE) cases and determine whether a correlation exists between ictal semiology signs, their localization/lateralization value after intracranial electroencephalography (EEG) monitoring, and surgical outcomes.
Methods: A retrospective study was conducted among epilepsy patients who underwent resective surgery for TLE or TPE after intracranial EEG monitoring between January 2008 and December 2018 at King Faisal Specialist Hospital in Riyadh, Saudi Arabia. Data were retrieved for 464 patients; 181 had intracranial electrode monitoring.
Results: Forty-eight patients with a mean age of 27 years (SD=8.4) were included; 15 patients had TPE. Auras were frequently reported, emotional auras, in the form of fear (35%). The localization/lateralization value of aura was statistically significant for TPE patients, including visual hallucinations and vertigo, lateralized to the left and right temporo-occipital, respectively (p=0.009 and <0.001). Early-onset ictal manual automatism, oral automatism, late-onset dystonic posture, and late head-turning were significant for TLE without significant lateralization value. The ictal onset zone’s localization was significant between the scalp and intracranial EEG findings in mesial TLE patients. The probability of seizure freedom (Engel class I) was 74%, 60%, and 67% at 2-year follow-up for mesial, lateral TLE, and TPE, respectively.
Conclusion: Our results are consistent with previous studies and confirm the importance of ictal semiology signs in TLE and TPE. The addition of intracranial EEG monitoring in these cases helped improve the surgical outcomes.
Epilepsy is one of the most common neurological disorders, affecting approximately 70 million people globally.1 Thirty percent of these patients have drug-resistant epilepsy,2 and most cases referred for epilepsy surgery involve temporal lobe epilepsy (TLE).3 However, after standard temporal lobectomy, around 40% of these patients will experience recurrent seizures.4 A variety of explanations have been proposed for these surgical failures, including incomplete removal of the epileptogenic zone, additional contralateral focus (bilateral TLE), dual pathology (mesial and neocortical), and extended epileptogenic focus to the neighboring structures, including extratemporal or temporal plus epilepsy (TPE).5
The TPE is defined as focal epilepsy with a complex epileptogenic network involving the temporal lobe and the surrounding areas, such as the orbitofrontal cortex, insula, operculum, and temporo-parieto-occipital junction.6 A thorough presurgical evaluation is required to delineate the epileptogenic zone for successful resective surgery. In phase I assessment, scalp video electroencephalography (EEG) monitoring, brain magnetic resonance imaging (MRI), and neuropsychological evaluation are needed. Further non-invasive investigations can be included if the initial results are discordant. To reach a well-demarcated epileptogenic focus requires intracranial monitoring, including the subdural grid, strips, and depth, which is known as phase II assessment.7 Seizure semiology is the first step in a presurgical evaluation, and ictal semiology and scalp-EEG results play a valuable role in distinguishing TLE from TPE.8 Patients with TLE are more likely to experience abdominal auras, ictal gestural automatism, and post-ictal amnesia. However, TPE patients are more likely to experience gustatory hallucinations, rotatory vertigo, auditory illusions, contralateral eye and head versions, piloerection, and ipsilateral tonic posturing. Similar findings were highlighted in a review of TPE cases.4 Furthermore, laryngeal and throat constriction and the atypical distribution of somatosensory symptoms at seizure onset have been found.4
Although some studies have found a correlation between seizure semiology and intracranial EEG monitoring in TLE (mesial vs. lateral) vs. TPE, none evaluated lateralization values. This study aims to utilize our tertiary center’s experience with TLE and TPE cases and determine whether a correlation exists between ictal semiology signs, their localization/lateralization value after intracranial electroencephalography (EEG) monitoring and surgical outcomes. We also highlight the process of phase I presurgical assessment (including ictal/interictal scalp EEG, MRI, positron emission tomography [PET], and neuropsychology) in our center.
Methods
Patients and data collection
We conducted a retrospective cohort study of epilepsy patients who underwent resective surgery for TLE or TPE after intracranial EEG recording between January 2008 and December 2018 at King Faisal Specialist Hospital and Research Center in Riyadh, Saudi Arabia. The hospital is a tertiary referring center for most drug-resistant epilepsy cases in Saudi Arabia. Data related to the treatment of 464 patients were retrieved from the hospital database for all epilepsy surgeries performed during our study-designated period. A total of 181 patients received intracranial electrode monitoring for drug-resistant epilepsy; 130 of these patients received extra-TLE surgery, and 51 underwent TLE or TPE surgery after intracranial EEG recording. Three patients were excluded; 2 had bilateral TLE with more than one ictal semiology, and one patient had incomplete data. The following clinical variables were studied: age, gender, handedness, age of epilepsy onset, number of years until surgical resection, seizure frequency per month, risk factors, and hospital stay length.
All patients had drug-resistant (DRE) epilepsy, as defined by the International League Against Epilepsy (ILAE) task force guidelines: the failure of adequate trials of 2 appropriately chosen and tolerated antiepileptic drug schedules, as either monotherapy or in combination, to achieve sustained seizure freedom.9 Patients were classified as having focal epilepsy with or without bilateral tonic-clonic seizures. Presurgical evaluation data were reviewed from the Epilepsy Monitoring Unit (EMU) and epilepsy conference reports, which were dictated after epilepsy case discussions by the epilepsy fellow and attending epileptologists. The consensus decision was made by a group of physicians, including adult/pediatric epileptologists, epilepsy surgeons, and neuropsychologists. Surgical resection details were reviewed from operative reports and post-op MRI/CT scan when available. Postoperative seizure control was ascertained via follow-up clinic visit documentation in the hospital’s electronic records system (ICIS) using Engel classification after 2, 5, and 10 years of follow-up.
Non-invasive evaluations
All patients were monitored with scalp video-EEG using an international 10–20 electrode system with additional temporal basal electrodes. A three-tesla MRI brain scan, fluorodeoxyglucose (FDG)-PET scan, and neuropsychological evaluation were performed for all subjects. The intracranial sodium amobarbital procedure, also known as the Wada test, was performed on 19 patients. Seizure semiology analysis was based on EMU and epilepsy conference reports; all included patients had one seizure semiology, which was focal with/without impaired awareness. Auras were classified according to the ILAE’s 201710 classification: autonomic (rising epigastric sensation, nausea, or palpitation), cognitive (Deja vu, dreaming states, depersonalization), emotional (fear or anxiety), and sensory (vestibular vertigo and dizziness, olfactory, gustatory, complex auditory and visual hallucination, and headaches). Ictal phase semiology consisted of motor symptoms (manual/oroalimentary automatism, tonic, clonic, limb dystonia, rhythmic ictal nonclonic hand [RINCH] movement, head-turning, face grimacing or contractions, or eye blinking) or non-motor symptoms (‘speech arrest or ictal speech’ and behavioral arrest). Aphasia, psychosis, cough, and vomiting were evaluated in the post-ictal phase.
Intracranial EEG evaluation
Intracranial EEG evaluation was indicated when discordant data were reported during a phase I presurgical assessment, including bitemporal onset on scalp EEG, lesion contralateral to the proposed hypothesis, large epileptogenic zone involving temporal and temporal plus regions, non-lesional MRI brain scans, old insults with large areas of encephalomalacia, and ‘redo’ surgery. Our epilepsy utilizes the intracranial implantation of the grid, strip, and depth electrodes by craniotomy or a burr hole. Intracranial EEG recordings with subdural (SD) grids, strips, or depth electrodes were performed on all subjects. However, the coverage was applied according to the proposed hypothesis. As indicated, e.g., bilateral temporal lobe strips (8x1 contacts for each strip) placed on the lateral temporal lobe (superior, middle, and inferior temporal gyri) and mesial temporal strips (4x1 contacts for each strip) placed 2 cm, 4 cm, and 6 cm from the temporal pole. Additionally, bilateral orbitofrontal, frontopolar, mesial frontal, operculum, temporo-occipital, and parietal regions were covered by strips (4x1 or 6x1 contacts for each strip) accordingly. Depth electrodes (8–10 contacts) were added bilaterally to the amygdala and hippocampus if required. Furthermore, depth electrodes (8–10 contacts) were placed over the insula. In some patients, additional strips or grids were used, especially for lesional and redo cases. After electrode implantation, patients returned to the EMU for monitoring. The EEG montages were created by anatomical localization (anterior-posterior) using bipolar, average, and referential montages. After capturing each patient’s habitual seizures (an average of 3–5 seizures), the ictal onset zone was analyzed by epilepsy fellows and the attending epileptologists. Then, an epilepsy case discussion was conducted to reach a consensus decision. Later, patients were returned for electrode removal and surgical resection. Postoperative surgical outcomes were evaluated using Engel’s classification system,11 and class I and II were considered good outcomes. Patients were followed for 2, 5, and 10 years.
Statistical analysis
Data were coded and analyzed using Statistical Package for the Social Sciences (SPSS) software, version 25. The descriptive analysis results for continuous variables were presented as the mean and standard deviation, whereas categorical variables were described using frequencies and percentages. Additionally, chi-square tests were used to assess the association between the outcome and independent variables. An independent Student’s t-test and one-way analysis of variance (ANOVA) were used to compare 2 or more means. The significance level was set at p≤0.05, with a confidence interval of 95%. Before conducting the study, ethical approval was obtained from the Institutional Review Board (IRB) at King Faisal Specialist Hospital and Research Center (# 2191 144).
Results
Demographics and risk factors
A total of 48 patients who underwent surgical resection for TLE or TPE were included in the study, as shown in Table 1. Fifteen patients had TPE (31%), and 33 had TLE (69%). Thirty-two patients were male (67%), and 16 (33%) were female. The mean age was 27 years (SD=8.4). The mean number of years of epilepsy before surgery was 15 years (SD=9.0). There were no statistically significant differences between the TLE and TPE groups in age, age of epilepsy onset, or seizure frequency per month. Epilepsy risk factors were studied in our population (i.e., mesial TLE vs. lateral TLE vs. TPE), including a history of family history of epilepsy (n=13, 27%), history of head trauma (n=10, 21%), febrile seizures (n=9, 19%), history of central nervous system (CNS) infections (n=2, 4%), and perinatal complications (n=1, 2%). A family history of epilepsy was statistically significant (p=0.049) in patients with mesial TLE.
- Demographic data.
Seizure semiology
Auras were frequently reported in both groups, and emotional auras in the form of fear were the most common presentation (35%). The localization/lateralization value of aura was statistically significant for TPE patients, including visual hallucinations and vertigo, lateralized to the left and right temporo-occipital, respectively (p=0.009 and <0.001, respectively). One patient reported pre-ictal headache (17%), and ictal speech was observed in 2 patients (33%) in the TPE group, with a right orbitofrontal seizure onset zone. The contralateral facial contraction was observed in both the TLE and TPE groups but was most frequently in the right orbitofrontal ictal-onset zone in 4 patients (67%). Early-onset ictal manual automatism, oral automatism (specifically, lip-smacking), late-onset dystonic posture, and late head-turning were statistically significant for TLE without significant lateralization value. A behavioral arrest was observed in patients with mesial TLE without significant lateralization value. Rhythmic ictal nonclonic hand movements were observed in contralateral temporal and temporal plus (orbitofrontal) subjects without significant localization or lateralization value. The progression to bilateral tonic-clonic seizure was statistically significant (p=0.046) in the left mesial TLE patients’ lateralization value. Detailed correlation between pre-ictal/ictal phase and ictal onset zone (IOZ) localization and lateralization value is shown in Table 2 & 3.
- Correlation between pre-ictal phase and IOZ localization and lateralization value.
- Correlation between seizure symptomatology and IOZ localization and lateralization value for the ictal phase.
Phase I pre-surgical evaluation
In phase I presurgical evaluation, scalp EEG recording was performed, which revealed spikes and sharp waves were the most common interictal epileptiform discharge (IIED). They were frequently lateralized ipsilaterally to the ictal onset zone. However, frontotemporal spikes were observed in orbitofrontal and mesial temporal groups without significant localization value. Bilateral IIED was recorded (n=16, 33%), whereas (n=6, 13%) had non-epileptiform discharges in the form of continuous/intermittent slow activity. The most frequent ictal pattern was a poorly localizing onset (n=12, 25%). These changes were more frequent in TLE subgroups than in TPE. The localization value for a scalp ictal onset zone over the mesial temporal lobe matched the intracranial EEG findings (n=15, 31%) (p=0.023). Rhythmic theta ictal onset was more consistent with mesial TLE than lateral TLE or TPE cases. The MRI of the brain showed lesional epilepsy (n=39, 81%) patients and non-lesional epilepsy in (n=9, 19%) patients. The MRI of the brain, FDG-PET scans, and neuropsychology assessment were lateralized to the lesion’s side in 82%, 81%, and 56% of patients, respectively. There was no statistical significance in the localization or lateralization value of PET and neuropsychology assessment in our sample. The Wada procedure was performed on 40% of the subjects. More detailed data regarding the TLE and TPE groups and their lateralization values are presented (Table 1).
Phase II pre-surgical evaluation
In phase II pre-surgical evaluations, the mean number of seizures recorded during intracranial EEG monitoring was 6 (SD=5.1). Seizure onset zones in our population, based on intracranial recording, were as follows. In the TPE group, the zones were right orbitofrontal (n=6, 13%), left orbitofrontal (n=4, 8%), right temporo-occipital (n=2, 4%), and left temporo-occipital (n=3, 6%). However, in the TLE group, the zones were right mesial temporal (n=10, 21%), left mesial temporal (n=10, 21%), bilateral mesial temporal (n=3, 6%), right lateral temporal (n=3, 4%), and left lateral temporal (n=7, 15%). Intracranial ictal onset EEG patterns were recorded in 26 patients with low voltage fast activity (54%), which was the most common (IOZ) pattern; patients had high amplitude spikes (n=2, 4%), high amplitude spike-and-slow wave discharges (n=2, 4%), rhythmic spike and sharp waves (n=6, 13%), rhythmic high amplitude polyspikes (n=3, 6%), sharp rhythmic waves (n=1, 2%), rhythmic polymorphic delta slowing (n=3, 6%), and periodic lateralized discharges (n=2, 4%).
Surgical outcomes
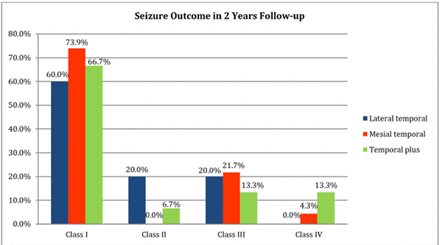
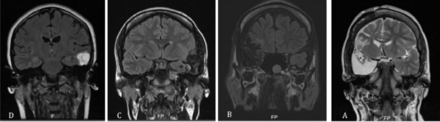
Surgical procedures performed for our subjects included; anterior standard temporal lobectomy (n=26, 54%), anterior standard temporal lobectomy with lesionectomy, orbitofrontal resection, or temporo-occipital resection (n=5, 10%), (n=9, 19%), (n=5, 10%), respectively. An awake craniotomy with intraoperative language mapping was done in one patient from the previous group. Resection for residual temporal neocortical and mesial structures was performed (n=3, 6%) (2 had redo surgery, and one had an encephalomalacia). The pathological changes in this population included gliosis (n=8, 17%), gliosis with marked increased heterotopic neurons in white matter and hippocampal sclerotic changes(n=9, 19%), marked neuronal loss and gliosis (n=6, 13%), and mild cortical dysplasia and gliosis (n=3, 6%). Two subjects (4%) showed cavernous hemangioma. Subpial (chaslin’s) gliosis and increased white matter heterotopic neurons were revealed in 8 patients. One showed sclerosis of the cornu ammonis (CA1) region. Focal cortical dysplasia (FCD) Ia, Ic, IIa IIb, IIIa, IIId, and DENT showed (4%, 2%, 2%, 4%, 2%, 2% and 2%), respectively. Three patients showed non-specific changes. The probability of seizure freedom (class I in Engel’s classification system) was 74%, 60%, and 67% at 2-year follow-up for mesial, lateral TLE, and TPE, respectively. Detailed data regarding surgical outcomes at the 2-year follow-up are presented in Figure 1. Moreover, the probability of seizure freedom was 58%, 31%, and 13% at 2-, 5-, and 10-year follow-up, respectively. Examples of cases with Engel class I with postoperative MRI are shown in Figure 2. The mean hospital stay was 20 days (SD=5.5). There were no subdural complications in our population, but one patient experienced an intraoperative anesthetic complication. There were no statistically significant differences between the number of years of epilepsy and surgical outcomes at the 2-year follow-up.
- Seizure outcomes in patients in the mesial, lateral temporal, and temporal plus groups at the 2-year follow-up. In our sample, 74%, 60%, and 67%, respectively, of mesial temporal, lateral temporal, and temporal plus epilepsy cases achieved class I in Engel’s classification system.
- Postoperative MRI scans of patients who achieved class I in Engel’s classification system at the 2-year follow-up. A) A 27-year-old left-handed patient with a ten-year history of epilepsy; the patient was found to have left middle temporal gyrus cavernoma. She reached Engel’s class I post-resection. B) A 22-year-old, right-handed patient with new-onset epilepsy and lesional MRI; suspected DENT vs. ganglioglioma. The pathologist confirmed DENT. At post-op follow-up, she was assessed as Engel class I. C) A 26-year-old, right-handed patient with a history of epilepsy; the patient was found to have cortical dysplasia on brain MRI and confirmed the pathologist as FCD IIa; the patient was seizure-free at post-op. D) A 20-year-old, right-handed patient with a history of DRE; the patient was found to have hippocampal sclerosis and dorsofrontal encephalomalacia, and the resection of temporal structures plus orbitofrontal resulted in seizure freedom.
Discussion
This study results support the use of seizure semiology as the first step in evaluating TLE and TPE cases. Furthermore, we demonstrated that semiology and scalp ictal/inter-ictal EEG findings could differentiate TLE from TPE. The complexity of epileptogenic networking in the TPE and its similarity in semiology to other lobes; makes intracranial recording the gold standard for evaluating these patients. Nevertheless, the completion of phase I presurgical evaluation provides more lateralization value through MRI, PET, and neuropsychology assessment to delineate the epileptogenic zone and focus on intracranial electrodes’ implantation. Intracranial EEG electrode coverage of the neighboring structures was performed in our TLE cases to rule out the neighboring structures’ involvement and improve surgical outcomes. Our results are consistent with the literature in finding no significant differences between TLE and TPE groups in age, age of epilepsy onset, or seizure frequency.8 Family history was the most frequent epilepsy risk factor and was significant for mesial TLE. In contrast to a previous review that the presence of febrile seizures during infancy was associated with drug-resistant mesial temporal epilepsy.12
Emotional aura in the form of fear was the most frequent aura in this study results, consistent with a previous study as the most frequently reported aura,13 followed by digestive symptoms (epigastric sensations). Both were commonly reported by TLE and TPE patients, with a higher frequency in mesial TLE but without significant statistical value.8 Previous reports concluded that an aura of fear indicates an amygdalar epileptic focus confirmed by intraoperative stimulation.13 Moreover, there was a lateralization significance for experiential phenomena to the language-dominant hemisphere.14 In this study, visual auras can differentiate TPE from TLE cases. Vestibular auras were reported by one patient in our sample who had TPE involving the temporo-occipital junction, which is in line with the literature8 and a reported case15 that was later confirmed by cortical stimulation of the precuneus of the non-dominant parietal lobe to elicit rotatory vertigo.16 Ictal motor signs, such as manual automatism and dystonic posture, were commonly observed in TLE and TPE.8 Gestural automatism, specifically lip-smacking, was associated with TLE, and this finding is similar to those reported in the literature.8 It was hypothesized to be caused by the involvement of mesiotemporal lobe structures and, less commonly, the neocortex.8 Similarly, our results suggest that gestural automatism presents more commonly in mesial temporal than lateral TLE cases.17 Motor manifestations in the form of ipsilateral early manual automatism, contralateral late dystonic posture, and late contralateral head-turning are more consistent with mesial than lateral TLE, concordant to the literature.18 However, these findings were not consistent in 67% of patients with unilateral versive eye and head phenomena with an ipsilateral medial TLE focus.19 Our data indicate that the progression to bilateral tonic-clonic seizures is significant in lateralization value with mesial TLE; however, it did not differentiate between TLE and TPE.8 Moreover, it showed similar lateralization significance.20 Post-ictal psychosis was more common in the TLE group, but a larger sample is needed to verify this result. Data regarding post-ictal amnesia were not collected in the current study, but it has been reported as significant in the TLE literature, especially with left-sided TLE.8 In this study, the presence of ictal or inter-ictal temporal spikes did not exclude TPE, as in previous reports.8 Furthermore, a similar conclusion can be drawn that even with focal anterior or infero-mesial epileptiform discharges, it does not exclude the possibility of extratemporal origin.21 The most frequent inter-ictal patterns were ipsilateral temporal spikes and sharp waves, consistent with the literature.8 Also, studies showed that TPE cases with bilateral inter-ictal abnormalities are associated with poor postoperative outcomes.22,23 In this study result, the most common ictal patterns were poorly localizing seizure onset in TLE subgroups; however, it contradicts other reports which were more consistent with the TPE group.8 It was found that ictal scalp EEG was significantly lateralized to unilateral inter-ictal spikes.24 In line with this study, the presence of ictal onset as rhythmic theta discharge was more frequently observed in mesial TLE than lateral TLE or TPE.18,25 The PET scans revealed lateralization value in most of our patients, in concordance to the epileptogenic zone defined by the intracranial monitoring, regardless of the MRI findings. Similarly, it was localized to the seizure onset zone’s lobe in 75% of the patients.20 The PET scan lateralization value showed good concordance with stereoencephalography (SEEG) findings.26
The rate of seizure freedom (class I of Engel’s classification system) was 58%, 31%, and 13% at 2-, 5-, and 10-year follow-up, respectively. However, it was lower than expected at the 5- and 10-year follow-up. This could be due to losing some patients to follow-up after two years and using a small population compared to other reports. Also, seven patients who did not achieve seizure freedom due to the conclusion of bilateral temporal lobe epilepsy (BITLE) with intracranial recording in three cases, lesional epilepsy with wide epileptogenic lesions in three cases; due to an old insult or an area of encephalomalacia, and one patient with tuberous sclerosis (TS) and multiple tubers that improved partially after removing the most epileptogenic focus. Furthermore, in a similar cohort study involving TLE and TPE cases, the probability of reaching Engel class I was 77%, 71%, and 67%, at 2-, 5-, and 10-years follow-up.27 Studies have addressed concerns about complications after intracranial recordings, such as subdural or epidural strips or grids; however, they are considered generally safe,28 but few studies have reported morbidity and mortality with depth electrodes.29 In our sample, no intracranial electrode-related complications were reported, but one patient experienced intra-operative anesthetic complications.
This study experience is contributing to the growing knowledge in TPE. However, our study’s major limitation is the small number of TPE cases, which did not include all TPE subgroups. Larger sample size and the inclusion of TPE cases with an operculum and insular lobe epilepsy are recommended. The lack of stereoencephalography (SEEG) investigation may ease the detection of deep brain structures and the lobes’ mesial part. Utilizing functional MRI (fMRI) with these patients and re-evaluating surgical failure cases would also be helpful.
In conclusion, our results are consistent with previous studies and confirm the importance of ictal semiology signs in TLE and TPE. The intracranial EEG monitoring in suspected TPE cases helped improve the patients’ prognosis and surgical outcomes. Auras of visual hallucinations and vertigo are consistent with TPE in temporo-occipital subgroups with significant lateralization value. Intracranial EEG monitoring is the gold standard in demarcating the epileptogenic zone and safely resecting it.
Copyright
Whenever a manuscript contains material (tables, figures, etc.) which is protected by copyright (previously published), it is the obligation of the author to obtain written permission from the holder of the copyright (usually the publisher) to reproduce the material in Saudi Medical Journal. This also applies if the material is the authors own work. Please submit copies of the material from the source in which it was first published.
Acknowledgment
The authors would like to thank the epilepsy coordinator, Ms. Amal Abujaber, at King Faisal Specialist Hospital and Research Center in Riyadh, Saudi Arabia, for her assistance in collecting all epilepsy surgical cases from 2008–2018.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received November 1, 2020.
- Accepted March 22, 2021.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.