ABSTRACT
Objective: To develop clinical practice guidelines based on evidence based medicine on the use of abortive and preventive therapies for managing migraine headaches. We formulated these guidelines to offer evidence-based recommendations to improve the knowledge of physicians, healthcare professionals, and policymakers in migraine headache management.
Method: A panel of 11 experts from different sectors in Saudi Arabia approved 26 questions on abortive and preventive therapies for migraines. To develop each question, we searched “PubMed” and “Cochrane Library” databases for recent relevant systematic reviews published between 2013 and 2024. We employed the Grading Recommendations, Assessment, Development, and Evaluation methodological approach to ensure the certainty of the collated evidence and to formulate the recommendations. The expert panel voted electronically on each recommendation, and a consensus was defined as >70% agreement.
Results: We formulated a total of 26 recommendations. Of these, 14 are focused on abortive therapy for acute migraine attacks, whereas 12 are focused on the prevention of episodic or chronic migraines. These guidelines strongly recommend the use of paracetamol and ibuprofen as the first-line treatment for mild to moderate migraine. Furthermore, we concluded that propranolol should be considered as the first-line preventive intervention for migraine.
Conclusion: The Saudi clinical practice guidelines offer systematically validated recommendations of migraine headaches in adults. The recommendations are potentially beneficial for all healthcare professionals managing patients with migraine headaches.
Migraine is a common disabling disorder that affects 14% of the global population.1 A previously published meta-analysis (MA) of 36 studies revealed that the prevalence of migraine in Saudi Arabia is 21%, indicating that the prevalence and burden of the disease in the country are high.2 The prevalence of migraine in the United States has remained relatively stable over the last 30 years, affecting approximately 15.9% of adults in 2018, with a higher prevalence in women (21%) than in men (10.7%). Despite the consistent prevalence of migraine headaches, the incidence of migraine-related disability has increased, with a growing proportion of individuals experiencing moderate to severe disability, as estimated using the Migraine Disability Assessment Scale (MIDAS). Migraine continues to be a significant public health issue, accounting for millions of emergencies and office visits annually, disproportionately affecting women of childbearing age. These statistics highlight the need for increased attention and funding for migraine treatment and research to lessen the burden of this chronic condition.3,4
A recent cross-sectional study of 2,316 Saudi adults showed that the mean frequency of migraine cases in Saudi Arabia is 3.5 days per month, with a mean symptom duration of up to 12.1 hours, mean symptom intensity of 2.4, and a migraine-associated health burden of approximately 1.5% of the total health status. Notably, study revealed no gender-specific differences in the primary symptom burden of the disease. In addition, the patients reported a 4.7% loss in the number of workdays.5
Migraine is a chronic condition that impacts the quality of life for many patients.6,7 Migraine patients may present with persistent moderate to severe headaches that may last from four to 72 hours in adults and are considered to have primary headache disorder.8 The pharmacologic treatment of migraine includes acute (i.e., abortive) and preventive (i.e., prophylactic) approaches, commonly used in patients experiencing recurrent severe headaches. Preventive therapy aims to decrease the duration, frequency, and severity of migraine headache attacks.9 Preventive therapy is typically used in patients suffering from four or more episodes of headache monthly or at least an average of 8 headache days monthly.10 Additionally, prophylactic interventions are recommended for several patient subgroups, including those who experience incapacitating episodes despite suitable acute treatment strategies, individuals with intolerance or contraindications to acute pharmacotherapy, patients presenting with medication overuse headache, those expressing a preference for preventive measures, and patients diagnosed with specific migraine variants, such as hemiplegic migraine, migrainous infarction, and those with frequent, persistent or uncomfortable aura symptoms.10
Previous guidelines for the treatment of migraine patients were published in the Kingdom of Saudi Arabia (KSA) in 2015.11 Our aim is to offer an updated and more comprehensive approach to develop updated recommendations for the pharmacological management of migraine headaches.
Scope and purpose
These guidelines offer recommendations regarding the pharmacological management of migraine headaches in adults. Specifically, these guidelines are focused on pharmacological abortive and preventive therapies for the management of migraines in adults but not on physical or psychological therapies, devices, or surgical interventions. These recommendations are not applicable to children or adolescents with migraines.
Goal
To provide evidence-based guidelines that can be utilized by healthcare professionals for the management of patients diagnosed with migraine.
Objectives
These Saudi Clinical Practice Guidelines offer practical guidance for healthcare workers treating individuals with migraines. The primary objectives of these guidelines are as follows:
To serve as a national reference on migraine clinical practice
To optimize abortive and preventive treatment for migraine
To improve the quality of migraine management
Guidelines scope
What are evidence-based recommendations for the management of migraine headaches among adults in KSA?
What are pharmacological interventions for the abortive and preventive therapies of migraine among adults in KSA?
End-users
The end users of these guidelines are neurologists, primary care and family medicine physicians, clinicians specialized in pain management, emergency and internal medicine, and clinical pharmacists in KSA. These guidelines provide valuable insights into the management of migraine for policymakers, researchers, and guideline developers.
How to use these guidelines
The Ministry of Health of KSA aims to provide clinicians and their patients with guidance for managing migraine headaches among adult patients of all genders.
Regarding other guidelines developed using the “Grading Recommendations, Assessment, Development, and Evaluation” (GRADE) approach, it is essential to recognize that no set of guidelines or recommendations can comprehensively cover every unique aspect of each patient’s case. Therefore, supervisors or administrators responsible for evaluating clinicians’ actions should avoid applying these recommendations rigidly or universally.
Each recommendation is accompanied by statements that consider underlying morals and preferences, resource usage, feasibility, equity, tolerability, and other relevant factors. These statements are essential for the accurate interpretation of the recommendation. We also strongly emphasize that the guideline does not replace sound clinical judgment in daily practice. Clinicians should also consider the patient’s individual needs and circumstances in choosing the best management approaches for adults with migraine in line with evaluating impacts on outcomes and risk-benefit ratio of the diagnostic and therapeutic means, as well as any relevant comorbidities or complications when applying the guidelines to clinical practice.
Methods
These guidelines were established using the GRADE methodological approach.12
Panel composition
The Ministry of Health in KSA in collaboration with the Saudi Society of Clinical Pharmacy compiled a panel of 11 experts in research methodology, neurology, headache disorders, pain medicine, family medicine, and clinical pharmacology. Geographical and gender balance were considered, whenever possible, during the selection of the panel members.
Group interaction and process
Group interaction in developing guidelines using the GRADE approach promoted transparency, consensus-building, and the integration of multiple viewpoints. This approach helped mitigate potential biases and ensured that recommendations were formulated based on the most recent evidence summaries. The panel participated in meetings where they collaborated on key elements of the guideline development process. These elements included creating methodological guidance, synthesizing evidence, assessing the inevitability of evidence, and formulating recommendations.
Selection of questions and outcomes’ prioritization
We reviewed recently published guidelines and abstracted Population, Intervention, Comparator, and Outcome (PICO) questions from the published guidelines.13 Subsequently, the panel discussed which PICO questions are important for clinical practice in KSA; they retained relevant questions and omitted questions that were deemed less relevant. Additionally, the panel was allowed to suggest new PICO questions. The guideline chairs reviewed and agreed on 26 questions. For each PICO, we used the GRADE approach to classify outcomes as critical or important.14 Through prioritization of outcomes encompassing the absence of pain at two hours post-intervention, the maintenance of analgesia at 24 hours, a minimum 50% decrease in the frequency of monthly migraine days, and the occurrence of treatment-related adverse effects.
Evidence synthesis
For each PICO question, we performed an electronic search, including “PubMed” and “the Cochrane Library” for relevant studies, with the assistance of 2 methodologists and a medical literature search expert. The specific search terms used for each PICO question are listed in (Supplementary Table S1). We retrieved all relevant systematic review (SR) and randomized controlled trials (RCTs) in these databases, covering the period from January 2013 to March 2024.
Three authors performed the “titles and abstracts” screening after checking the retrieved citations and included studies that met the criteria for each PICO question.
We aimed to include recent systematic reviews. If systematic reviews were not available or were outdated, we included RCTs addressing the corresponding PICO question. For RCTs, we used the “Cochrane Collaboration tool” to evaluate the bias within the included studies.15
We used the GRADE approach to determine the certainty of the available evidence for each outcome. Certainty of the evidence in the following domains was classified as “high,” “moderate,” “low,” or “very low”: risk of bias, publication bias, consistency in the findings, indirectness of evidence, and imprecision of the estimate.12
Assessing the certainty of evidence
For each PICO question, we used the GRADEpro guideline development tool14 to generate evidence profiles containing critical and important outcome absolute and relative effects and certainty assessment. In addition, We employed the GRADE to evaluate the quality and reliability of the evidence and to make clinical practice recommendations for migraine. The GRADE approach is widely used to assess the certainty of evidence used for making clinical practice recommendations. The GRADE approach assesses 5 components to estimate the overall certainty of evidence. The 5 components are “risk of bias,” “publication bias,” “imprecision,” “inconsistency,” and “indirectness.” We summarized the results and certainty of evidence assessment using evidence profiles.
After assessing the 5 domains of GRADE, the certainty of evidence was categorized as very low, low, moderate, and high.16 High certainty of evidence indicates “strong confidence that the true effect is close to the estimated effect.” Moderate certainty evidence indicates “moderate confidence in the effect estimate, with the true effect likely being close to it.” Meanwhile, the low certainty evidence implies that “the effect estimate is limited, and the true effect might differ significantly.” Finally, very low certainty evidence indicates that “the effect estimate is highly uncertain, recommending further research to reduce this uncertainty.” The strength of the suggestions is categorized as “strong” or “conditional”. Understanding the implications of the recommendation’s strength is crucial for informed decision-making (Table 1).
- Implications of recommendation’s strength.
- Strength and level of evidence for the recommendations for the abortive treatment.
- Strength and certainty of evidence for the recommendations on the preventive therapy and complementary and alternative medicine.
- Recommendations for treatment strategy of migraine.
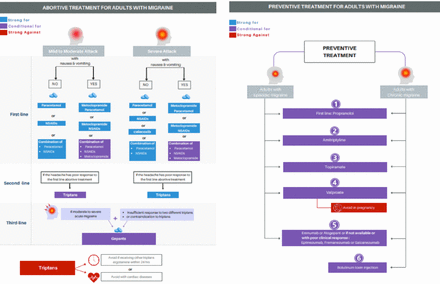
- Recommendations for treatment strategy for adult migraine patients; a) Abortive Treatment for Adults with Migraine; b) Preventive treatment for adults with migraine.
Medication cost
The pricing data for the medication discussed in the recommendation was collected and reviewed up to October 2024.
Results
Abortive Treatment
Question 1: Should paracetamol (acetaminophen) vs. no treatment be used for mild-to-moderate pain relief of migraine?
Recommendation 1: For the treatment of mild-to-moderate migraine pain, we recommend using paracetamol (acetaminophen) over no treatment (strong recommendation, moderate certainty).
Rationale
This recommendation was made according to the results of five systematic reviews and 115 RCTs (n=28,803), of which 6 RCTs (n=366) assessed the pain with the International Classification of Headache Disorders (ICHD) for migraine headaches.17 The results of these studies indicated that paracetamol improved freedom from pain (relative risk [RR]=1.89, [1.24 to 2.86]; high certainty, Table S2) and pain relief (RR of 1.61, [1.33 to 1.95]; high certainty, Table S2) at 2 hours.17 Additionally, another systematic review of 10 RCTs (n=2769) documented that compared to placebo, paracetamol improved the outcome of freedom from pain at 2 hours post-treatment, as well as alleviation of headache at 1 and 2 hours following intervention without increasing the risk of adverse events among patients with migraine.18
The guideline panel estimated that the paracetamol has a low direct cost per dose, in addition to a low cost per treatment episode or multiple episodes. Paracetamol is widely available, affordable, has a very good safety profile, and is commonly used by patients of all income groups and diverse backgrounds. Hence, after discussion among panel experts, the guideline panel suggested that paracetamol use is acceptable and feasible.
Considering the low cost, high effectiveness, and accessibility of paracetamol for relief of migraine pain, its use is likely to reduce barriers to effective migraine treatment across diverse income groups and populations. Hence, the guideline panel issued a strong recommendation for the use of paracetamol for the management of mild-to-moderate migraine attacks. This recommendation is consistent with previously published international guidelines.13
Question 2: Should ibuprofen vs. no treatment be used for mild-to-moderate migraineurs?
Recommendation 2: For the treatment of mild-to-moderate migraine pain, we recommend using ibuprofen over no treatment (strong recommendation, moderate certainty).
Rationale
This recommendation was made according to an SR and MA of nine RCTs involving 4373 participants and 5223 migraine attacks. The study was focused on the use of self-administered ibuprofen for the management of migraine episodes. The aim was to assess the effectiveness and safety profile of ibuprofen, administered as monotherapy or combined with an antiemetic agent, in comparison to placebo and alternative therapeutic options for the acute management of migraine headaches in adult patients.19
Data from the study indicated that the use of ibuprofen 200 mg resulted in freedom from pain at 2 hours post-intervention compared to placebo treatment (RR=1.96, [1.36 to 2.81]; high certainty, Table S3) compared to placebo. Additionally, findings from six RCTs indicated that ibuprofen (400 mg) led to pain freedom at 2 hours (RR= 1.91, [1.60 to 2.28]; high certainty, Table S3) compared to placebo. Furthermore, four studies demonstrated that 2 doses of ibuprofen (400 mg) provided superior sustained headache relief over 24 hours compared to placebo (RR=2.17, [1.76 to 2.69]; high certainty, Table S3).19
Furthermore, 4 studies demonstrated that both doses of ibuprofen (200 and 400 mg) showed superior effect compared to placebo in relieving associated symptoms like nausea at 2 hours (RR=1.54, [1.27 to 1.86], and RR=1.33, [1.06 to 1.67]; high certainty, Table S3) and ibuprofen (400 mg) further provided relief from vomiting (RR=1.53, [1.21 to 1.92]; high certainty, Table S3) compared to placebo.19
Another MA demonstrated that the use of ibuprofen led to little/no difference in adverse events (RR=0.94, [0.80 to 1.10]); common adverse events with ibuprofen included nausea, dyspepsia, dizziness, dry mouth, and drowsiness.20 Migraine treatment with ibuprofen is affordable as a single 400 mg dose. Although no studies have specifically evaluated the cost-effectiveness of ibuprofen in KSA, the guideline panel considers it a cost-effective option. Ibuprofen is acceptable and feasible, with a likely positive impact on health equity. Furthermore, it is affordable, effective, and available in various dosages in KSA markets. It can also be administered as an over-the-counter drug.
The guideline panel concluded that the profits of ibuprofen significantly surpass the risks at the relief of pain. The evidence strongly supports its efficacy, highlighted by a dose-response curve with a low certainty of mild side effects. This recommendation is congruent with other international guidelines.13
Question 3: Should celecoxib vs. no treatment be used for treating migraine attacks in adults?
Recommendation 3: For the treatment of moderate-to-severe migraine pain, we suggest using celecoxib over no treatment (strong recommendation, moderate certainty).
Rationale
This recommendation was based on a phase III, RCT (1:1), conducted to estimate the efficacy of celecoxib oral solution for the treatment of moderate-to-severe pain in a single migraine attack.21 The 2-hour post-dose pain-free response rate was significantly higher in the celecoxib group compared to the placebo, with an estimation of 32.8% vs. 23.5% (p=0.020). For 2 hours post-dose, response rates of freedom from the most bothersome migraine symptoms (BMS) were significantly higher in the celecoxib (58.1% vs 43.9%, p=0.003) compared to placebo (moderate certainty, Table S4).21 Furthermore, a post hoc analysis indicated that celecoxib at dose of 120 mg was superior to placebo regarding pain and BMS freedom, as well as pain relief over 2 hours post-dose.22 In addition, the efficacy of celecoxib was supported by a recent RCT, which indicated a more effective intervention in terms of pain and BMS freedom among patients with migraine attacks at any time of pain or intensity.23
Adverse events were observed in 10.7% of patients treated with celecoxib oral solution and 9.9% of a placebo group. Dysgeusia was the most common side effect; however, no severe or drug-related adverse events that may lead to withdrawal were identified.22 On the other hand, it was found that celecoxib provides acute migraine pain relief with similar or fewer cardiovascular related and gastrointestinal-related events compared to previous interventions.24
Celecoxib shows promise as a cost-effective option for migraine treatment. The guideline panel suggested that celecoxib is acceptable, feasible, and its impact on health equity is likely to increase. Furthermore, the drug is available in KSA, is affordable, and has proven efficacy in treating migraine headaches among adults.
Question 4: Should sumatriptans vs. no treatment be used for the treatment of moderate to severe migraine?
Recommendation 4: For the treatment of moderate-to-severe migraine pain, we suggest using sumatriptan over no treatment (conditional recommendation, low certainty).
Rationale
An SR and MA of 64 RCTs (n=46,442) showed that sumatriptan increases the chances of being free from pain for at least 2 hours when compared to placebo (OR=3.46, [2.83 to 4.23]; moderate certainty, Table S5).25 Additionally, sumatriptan was potentially more effective for 2-hour pain relief at the 10 mg nasal spray dose (odds ratio [OR]=4.09, [1.43 to 11.71]; very low certainty, Table S5), with 221 more patients achieving pain relief per 1,000 compared to placebo. The effect was consistent across varying sumatriptan doses (10, 50, and 100 mg) and showed a dose-response gradient.26,27 A comprehensive network meta-analysis (NMA) of thirty-three studies evaluated various migraine treatments, with significant findings across different time points. At one hour post-dose, subcutaneous sumatriptan showed hight clinical disability relief (RR=3.11, [2.36; 4.10]) and nausea relief rates (RR=1.85, [1.08; 3.17]), and IV valproate led in phonophobia relief (RR=3.99, [1.66; 9.61]). Subcutaneous sumatriptan demonstrated highest rates in headache relief (RR=2.71, [2.36; 3.11]), phonophobia relief (RR=2.03, [1.35; 3.04]), and photophobia relief (RR=2.13,[1.50; 3.03]). Regarding safety, subcutaneous sumatriptan showed higher total adverse events compared to placebo but maintained comparable rates of serious adverse events and withdrawal due to adverse events.28
Sumatriptan probably increases the risk of adverse events compared with placebo (Table S5).25 A pooled analysis of RCTs differentiated between two categories of adverse events following oral sumatriptan (100 mg) administration. which includes nausea and malaise, likely represents migraine symptoms. The second category, including fatigue, sedation, and weakness, is likely the true side effects of the medication and typically occur during the recovery period.29 Additionally, an RCT showed that 26% of patients who received sumatriptan exhibited adverse events, including gastrointestinal symptoms, dizziness, and drowsiness.30
The guideline panel indicated that using sumatriptan is acceptable and feasible and will probably result in moderate savings; however, there are no cost-effectiveness studies in KSA. Furthermore, the panel determined that there is little impact on health equity. Overall, the guideline panel issued a conditional recommendation for using sumatriptan versus no treatment, emphasizing the priority of achieving significant pain relief despite the potential for mild but common adverse effects. This recommendation is consistent with other international guidelines.13
Question 5: Should rizatriptan (vs. no rizatriptan) be used within 24 hrs of using ergotamine or another triptan for the treatment of adults with migraine?
Recommendation 5: For the treatment of acute migraine in adults receiving other triptans or ergotamine within 24 hours, we recommend against using rizatriptan (strong recommendation, very low certainty).
Rationale
No study has been conducted to specifically investigate the use of rizatriptan versus not using rizatriptan for migraine relief in the context of using ergotamine or other triptans. Consequently, this recommendation was based on theoretical evidence (very low certainty, Table S6).31
The co-administration of triptans with either ergot alkaloids or monoamine oxidase inhibitors has the potential to induce serotonin syndrome, a serious adverse drug reaction resulting from excessive serotonergic activity.31 However, there is no significant evidence to confirm the assumption about serotonin syndrome from triptans alone.32 Although a study on healthy subjects failed to show worsening of vasoconstriction with co-administration of rizatriptan and ergotamine, given that both are vasoconstrictors, the lack of studies assessing the safety of this combination and the availably of safer alternatives that do not carry the risk of ischemic complications; the guideline panel issued a strong recommendation against using rizatriptan in adults who have received ergotamine or other triptans within 24 hours. This recommendation, congruent with other international guidelines,13 values avoiding significant harm despite very low certainty of evidence.
Question 6: Should metoclopramide vs. no metoclopramide be used for adults with acute migraine attacks accompanied by nausea and/or vomiting?
Recommendation 6: For the treatment of acute migraine attacks in adults with nausea and/or vomiting, we suggest using metoclopramide versus not using metoclopramide (conditional recommendation, low certainty).
Rationale
We identified a recent NMA of 16 RCTs (n=1934)33 that showed increased odds of being pain-free at 2 hours with metoclopramide use (OR= 4.92, [1.34 to 18.07]; very low certainty, Table S7). Additionally, metoclopramide reduced the need for rescue medications within the first hour (OR= 0.27, [0.15 to 0.49]; low certainty, Table S7).33
In addition, the findings of a recent study showed that while metoclopramide reduced migraine pain from initial levels when measured one hour after taking it, the results didn’t clearly show whether it was as good as, or worse than, sumatriptan for migraine pain relief. This means that they couldn’t draw definitive conclusions about how these 2 treatments perform in comparison.34
Several studies demonstrated that metoclopramide had a similar risk of side effects in comparison to placebo.35,36 Several side effects have been reported with metoclopramide use, such as drowsiness or light sedation, dizziness, nausea, dysphoria, restlessness, and flushing. In addition, extrapyramidal effects such as dystonia or akathisia were reported.37 However, a previous MA of 14 studies (n=1661) concluded an uncertain effect of metoclopramide on adverse effects with placebo (OR=0.92, [0.31 to 2.74]).37
The panel demonstrated that the use of metoclopramide is associated with moderate savings, rendering metoclopramide an affordable option for acute migraines in KSA. However, injectable metoclopramide is limited to hospital settings, restricting at-home use. Furthermore, the guideline panel determined that the balance of desirable and undesirable effects was probably favoring metoclopramide use and, therefore, issued a conditional recommendation for its use in patients with acute migraine with nausea and/or vomiting. This recommendation is congruent with other international guidelines.13
Question 7 Should eletriptan vs. no treatment be used for migraine relief be avoided within 24 hrs of using ergotamine or another triptan?
Recommendation 7: For the treatment of acute migraine in adults receiving other triptans or ergotamine within 24 hours, we recommend against using eletriptan (Strong recommendation, very low certainty).
Rationale
Prior studies specifically have not investigated the use of eletriptan compared to a placebo for migraine relief concerning avoiding ergotamine or another triptan within 24 hours. Therefore, this recommendation is based on theoretical evidence (very low certainty, Table S8).38
Theoretically, the coadministration of ergotamine with another vasoconstrictor may result in an additive effect. Hence, It is advisable to refrain from concomitant administration of sumatriptan and ergotamine-containing or ergot-derivative medications, such as dihydroergotamine or methysergide, within a 24-hour period, to minimize the risk of potential adverse interactions.38
Eletriptan is a highly selective serotonin 5-HT(1B/1D) receptor for the management of acute migraine headache.39 A previous study demonstrated eletriptan’s superior efficacy, the onset of action, and acceptability among patients in treating acute migraine compared to placebo at selected doses (20 mg, 40 mg, and 80 mg).40 It is worth mentioning that despite the lack of RCTs, evidence suggests that due to the potential risk of serotonin syndrome, the administration of eletriptan is strictly contraindicated in patients who have consumed any other 5-HT1 agonist, ergotamine-containing, or ergot-derivative medication within the preceding 72-hour period, due to the increased risk of severe adverse reactions.39
The guideline panel indicated that eletriptan use is associated with significant savings. Furthermore, the guideline panel judged that eletriptan is probably acceptable and feasible. According to the guidelines, eletriptan is used in KSA, which prohibits its concomitant use with other triptan within 24 hours, and it is likely to have no impact on health equity.
Overall, it is recommended to avoid using eletriptan within 24 hours of using ergotamine or other triptans. This precaution is recommended owing to the theoretical risk of serious cardiovascular adverse events and the availability of different alternatives.
Question 8: Should rimegepant vs. no rimegepant be used for the treatment of moderate-to-severe migraine?
Recommendation 8: For the treatment of moderate-to-severe migraine, we suggest using rimegepant over no treatment (conditional recommendation, high certainty).
Rationale
Our search identified an MA of three RCTs (n=3827), rimegepant, compared to placebo, resulted in higher odds of achieving pain freedom at 2 hours (OR= 2.10, [1.69 to 2.59]; high certainty, Table S9), pain relief at 2 hours (OR=1.93, [1.65 to 2.25]; high certainty, Table S9), and sustained pain freedom at 24 hours (OR=2.88, [1.74 to 4.78]; high certainty) no rimegepant 1.27, [1.01 to 1.60]; high certainty), which translates to 28 more events per 1000 (Table S9).41
The guideline panel suggested acceptable cost and savings are associated with rimegepant. The guideline panel indicated that rimegepant is likely acceptable, feasible to use in clinical practice, and has likely no impact on health equity. Thus, the guideline panel issued a conditional recommendation for using rimegepant as an abortive therapy compared to no treatment.
Question 9: Should ubrogepant vs. no treatment be used in treatment of moderate to severe headache?
Recommendation 9: For the treatment of moderate to severe migraine, we suggest using ubrogepant over no treatment (conditional recommendation, low certainty).
Rationale
Our search identified an SR and NMA of seven RCTs (n=12,859).42 The NMA regarding the efficacy of two hours of pain freedom demonstrated that ubrogepant (25 mg) and (50 mg) doses were significantly better than placebo (OR=1.59, [1.03 to 2.47]; high certainty; and OR=1.72 [1.22 to 2.41]; high certainty; respectively). Furthermore, ubrogepant (100 mg) showed higher efficacy than placebo (OR=2.0, [1.45 to 2.75]; low certainty, Table S10).42 Unlike higher doses, ubrogepant at 25 mg had little to no effect on continuous pain relief at 24 hours compared to placebo.42
In another trial that enrolled adults with migraine, ubrogepant use, compared to placebo, yielded higher rates of pain relief at 2 hours with 50 mg and 25 mg doses (50 mg: 21.8%; 25 mg: 20.7% vs. placebo: 14.3%).43
All ubrogepant doses (25 mg, 50 mg, and 100 mg) increased nausea and drowsiness compared to placebo or no treatment. For instance, 100 mg ubrogepant resulted in 25 nausea events per 1,000 people and 16 drowsiness events per 1,000 people. Dizziness was rare and not substantially different between the two groups.42
The guideline panel estimated that ubrogepant cost is acceptable. The panel indicated that ubrogepant was more affordable than lasmiditan and less affordable than rimegepant, sumatriptan, and eletriptan. Moreover, the guideline panel considered ubrogepant as likely acceptable, feasible, and with no impact on health equity. Therefore, the guideline panel issued a conditional recommendation for using ubrogepant as an abortive therapy compared to no treatment.
Question 10: Should eletriptan be used for moderate to severe pain relief of migraine?
Recommendation 10: For the treatment of moderate to severe migraine pain, we recommend using eletriptan over no treatment (strong recommendation, high certainty).
Rationale
Our search identified an SR and NMA of 64 RCTs (n=46,442) that examined the efficacy and safety of pharmacologic agents in acute migraine treatment.25 Eletriptan at 20 mg dose resulted in higher odds of achieving pain freedom at 2 hours compared to placebo (OR=3.15 [2.3 to 4.23]; high certainty, Table S11). In addition, there was a clear dose-response gradient with higher dosing, resulting in a larger effect (Table S11). In addition, eletriptan use resulted in higher odds of pain relief at two hours compared to placebo (OR=3.08, [2.29 to 4.15]; high certainty; Table S11).25
Another MA illustrated that eletriptan is one of the best triptans for acute migraine.44 Additionally, an MA found that eletriptan (40 mg) was superior to placebo for pain-free state and headache response over 2 hours (OR=4.95, [3.75 to 6.59], and OR=4.69, [3.91 to 5.59]) and similar results were observed in 24-hour sustained pain-free and headache response (OR=3.66, [2.63 to 5.15] and OR=3.65, [2.76 to 5.10]).45
Adverse events were slightly higher among patients receiving several doses of eletriptan than placebo, though not statistically significant (OR=1.19, [0.69 to 2.06]). Eletriptan (20 mg) had an absolute effect of 2 additional events per 1000 compared to placebo. Furthermore, eletriptan (40 mg) had an absolute effect of 3 more events per 1000 than placebo (OR=1.32, 95% CI [0.96 to 1.80]).25
An additional trial found that adverse events per attack were low for eletriptan 40 mg and 80 mg, the most reported adverse events were asthenia (5.0%) in eletriptan (40 mg) and asthenia (10%), followed by nausea (5.8%) in eletriptan (80 mg). Moreover, the incidence of severe side effects was lower in eletriptan (40 mg) compared to placebo (1.8% vs. 2.9%, respectively).46
The guideline panel judged the cost of eletriptan to be negligible and considered it a saving. It is estimated that eletriptan is probably acceptable and feasible and has no effect on health equity. This recommendation is consistent with other international guidelines.13
Question 11: Should lasmiditan vs. no treatment be used for moderate-to-severe migraine attacks?
Recommendation 11: For the treatment of moderate to severe migraine attacks, we suggest against using lasmiditan (conditional recommendation, moderate certainty).
Rationale
The recommendation was based on two meta-analyses of RCTs and a comparative disproportionality analysis.47–49
Paraesthesia risk showed a dose-response gradient with various lasmiditan doses (50, 100, and 200 mg) with RRs of 0.33, 1.39, 1.57, and 2.20, respectively (moderate certainty, Table S12).47 Additionally, lasmiditan can cause dizziness (9%–17%), drowsiness (6%–7%), and weariness (4%–6%).48
An MA demonstrated that lasmiditan use was substantially linked with a greater rate of pain freedom at two hours comparison with placebo (31.60% vs 17.55%) with an (RR=1.80, [1.34 to 2.42]), and the absence of the most unpleasant symptoms (42.82% vs. 30.38%).50 High doses of lasmiditan (100, 200, and 400 mg) produce paraesthesia compared to placebo.47 Lasmiditan was compared to triptans for migraine therapy in a WHO database analysis using IC. Both triptans and lasmiditan had a small risk of euphoric mood and hallucinations (IC 3.5, [2.9 to 4.0];, [4.5 to 5.6]; low certainty).48
Moreover, lasmiditan was associated with a higher likelihood of adverse effects compared to placebo: dizziness (OR=6.54, [4.24 to 10.07]), paraesthesia (OR= 4.28, 95% CI [2.97 to 6.17]), and fatigue (OR=5.67, [3.78 to 8.52]).49 Four RCTs indicated that lasmiditan improved pain-free status at 2 hours (R =1.74, [1.47 to 2.07]; high certainty) and at 24 hours (RR=1.55, [1.16 to 2.07]; high certainty) compared with the control group.51
Lasmiditan has been labeled by the FDA as a Schedule V controlled substance. It may cause central nervous system adverse reactions including significant driving impairment for up to 8 hours after each dose, serotonin syndrome, and cognitive and/or neuropsychiatric adverse reactions, including euphoria and hallucinations in about 1% of patients.52
The guideline panel deemed lasmiditan to be probably neither acceptable nor feasible due to its safety profile and side effects; however, the panel indicated that lasmiditan probably has no impact on health equity.
Question 12: Should valproate vs. ibuprofen be used for treating intractable and status migrainosus?
Recommendation 12: For the treatment of intractable and status migrainosus, we suggest using valproate over ibuprofen (conditional recommendation, low certainty).
Rationale
This recommendation was based on the only head-to-head prospective RCT study comparing the efficacy of valproate and ibuprofen among 99 patients with an acute headache who met migraine criteria.53 The study assessed the efficacy of “a single dose of 800 mg sodium valproate and 800 mg ibuprofen in 150 mL of normal saline” in treating intractable and status migrainosus. Changes in pain levels were evaluated using the numerical rating scale (NRS). The study indicated that the mean decrease in NRS values over 2 hours and 1 hour was significantly higher among the sodium valproate group with the ibuprofen group (mean difference [MD]=3.92, [3.67 to 4.46]; low certainty, Table S13), and (MD=3.61, 95%CI [2.96 to 4.26]; moderate certainty, Table S13) respectively. Furthermore, the findings illustrated that number of patients with pain relief was significantly higher among the sodium valproate group compared to the ibuprofen group (low certainty, Table S13).53
The guideline panel indicated that valproate use is acceptable and feasible. They judged that its impact on health equity is likely to be increased; the efficacy of valproate versus ibuprofen is much greater with comparable side effects.
Overall, they judged the balance between desirable and undesirable effects and likely favour valproate. However, further research is recommended to illustrate the adverse events associated with intravenous valproate and ibuprofen.
Question 13: Should intravenous valproate vs. dexamethasone be used for treating acute migraine in the emergency department?
Recommendation 13: For the treatment of acute migraine in the emergency department, we suggest using either valproate or dexamethasone (conditional recommendation, very low certainty).
Rationale
This recommendation was based on an MA of seven double-blinded RCTs (n=682)54 The MA’s findings revealed that IV valproate achieved comparable headache relief to dexamethasone (OR =0.38, [0.09 to 1.60]), and the need for rescue medications at one-hour post-administration was similar between IV valproate and dexamethasone (OR=3.35, [0.63 to 17.74]).
Finally, regarding headache recurrence, IV valproate demonstrated a comparable rate to dexamethasone (OR=1.04, [0.34 to 3.23]; very low certainty, Table S14).54 Another RCT demonstrated that intravenous sodium valproate (400 mg) showed similar effects to dexamethasone in treating acute migraine headaches.33
On the contrary, a trial conducted among patients with acute migraine headaches revealed that sodium IV valproate was found to be at least as effective as the comparator in treating acute migraine attacks based on visual analog scale (VAS) measurements. The severity of headaches reduced from 8.20 (7.72, 8.68) before treatment to 3.66 (2.99, 4.33) at 2 hours after treatment among patients who received sodium valproate. Similarly, among patients who received dexamethasone, the severity of headaches decreased from 8.46 (8.05, 8.86) before treatment to 3.59 (2.84, 4.35) at 2 hours after treatment.55
Regarding adverse events, a study illustrated that the odds of occurrence of adverse events among those patients who received IV valproate were 3.08 compared to dexamethasone (OR=3.08, [0.12 to 77.80]); however, the results were not significant.56
The guideline panel deemed the cost to be negligible and considered it a saving. Moreover, the guideline panel estimated IV valproate to be probably acceptable and feasible because it is effective with fewer side effects (100 mg dose) and is available in the KSA markets. In addition, the panel also considered that it would probably have no impact on health equity.
Intravenous valproate was comparable to dexamethasone in terms of headache relief rate, headache recurrence, and need for rescue therapy. However, some adverse events were reported with IV valproate. Despite the above-mentioned side effects, the guideline panel concluded that the balance between desirable and undesirable effects does not favour either IV valproate or dexamethasone. This assessment emphasizes the tolerable side effect profile of IV valproate.
Discussion
These Saudi national guidelines present recommendations for acute and prophylactic management of episodic and chronic migraines in adults. These recommendations provide clinical guidance for patients suffering from migraine, considering the specific characteristics of the Saudi population and healthcare systems. These recommendations were developed by a comprehensive literature review, considering the different abortive and preventive treatments and the decision-making process for implementing those treatments. Therefore, the current clinical practice guidelines consist of 26 recommendations covering abortive, preventive, and complementary therapies for episodic and chronic migraines among adults.
The goal of prophylactic treatments is to decrease the frequency of migraines by at least 50% without causing significant side effects. Abortive treatments prioritize the reduction and alleviation of migraine episodes. Paracetamol and ibuprofen are the preferred initial therapies for mild to moderate migraines, as there is substantial evidence confirming their effectiveness and safety. Nevertheless, it is advisable to refrain from using ibuprofen during the later stages of pregnancy due to the possibility of a negative impact on the fetus. Triptans, such as eletriptan and sumatriptan, are efficacious for treating migraines of moderate to severe intensity. However, they should not be used in individuals with specific cardiovascular and cerebrovascular disorders due to potential adverse effects. Celecoxib has comparable effectiveness to nonsteroidal anti-inflammatory drugs (NSAIDs) while offering better gastrointestinal tolerance. However, it is associated with an elevated risk of cardiovascular events. Rimegepant is a recommended choice for treating moderate to severe migraines, particularly in people with cardiovascular disease. Lasmiditan is not recommended due to its low effectiveness and probable adverse effects. Metoclopramide is prescribed as the medication of nausea and vomiting associated with migraines. Valproate and dexamethasone are equally effective in treating severe migraines in emergency situations. Amitriptyline has the potential to be used as a preventive treatment. However, it is important to carefully consider the benefits it offers in comparison to the potential risks of experiencing unpleasant effects. Supplementing with vitamin D may be advantageous for individuals with chronic migraine and insufficient levels of vitamin D.
It is important to continue implementing these Saudi National Clinical Guidelines by sharing them with national scientific societies, incorporating them into meetings and educational activities for health care professionals, and developing additional implementation strategies, including how well the recommendations are followed. However, some limitations must be considered when referring to these guidelines. The evidence that supports several recommendations was not as high as required. Hence, these recommendations must be updated as new evidence and therapeutic options emerge. Additionally, some strong recommendations were based on a low or moderate certainty of evidence. However, all the strong recommendations were backed by a high level of agreement, with the agreement levels being equal to or exceeding 80%.
Conclusion
These Saudi National Clinical Guidelines provide recommendations based on the latest evidence regarding abortive and preventive therapy for migraines in adults. These recommendations are expected to support healthcare professionals and policymakers in the KSA who are involved in the management of migraines. In addition, these recommendations will contribute to the optimization of treatment and improvement of the quality of care for patients with migraines.
The implementation of the guideline
It’s planned to incorporate migraine guidelines into national health policies, share them with national scientific societies, and encourage healthcare professionals to use them as guidance during migraine treatment and prevention journey. Moreover, these guidelines will be available online for all healthcare professionals to ease the implementation. Feedback regarding the implementation could be considered in the future to estimate the beneficial effect of the guidelines on the patient’s as well as their impact on the healthcare system.
Acknowledgment
The authors would like to express their deep gratitude to Riyadh Home Company and its dedicated team members who worked tirelessly on this project. Their expertise and commitment were invaluable in the successful completion of this manuscript. We also would like to extend our gratitude to SAGE author services for their effort in editing the English language for our manuscript.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.
- 58.
- 59.
- 60.
- 61.
- 62.
- 63.
- 64.
- 65.
- 66.
- 67.
- 68.
- 69.
- 70.
- 71.
- 72.
- 73.
- 74.
- 75.
- 76.
- 77.
- 78.
- 79.
- 80.
- 81.
- 82.
- 83.
- 84.
- 85.
- 86.
- 87.
- 88.
- 89.
- 90.
- 91.