ABSTRACT
Objectives: To investigate the potential utility of the C-reactive protein-to-albumin ratio (CAR) and the systemic immune-inflammatory index (SII) as a biomarker in distinguishing between BPPV and acute cerebellar infarction (ACI) due to posterior inferior cerebellar artery (PICA) involvement.
Methods: The data of 2545 patients registered in our hospital database between 2017 and 2024 with a diagnosis of vertigo were retrospectively analyzed and 102 patients with benign paroxysmal positional vertigo (BPPV) and 100 patients with ACI were included in the study. Mann-Whitney U test, Chi-square test, or Fisher’s exact test were used to compare variables between the two groups. Receiver operating characteristic (ROC) curve analysis was performed to investigate the predictive value of the data.
Results: The CAR and SII values were significantly higher in the ACI group compared to the BPPV group (p<0.001, p<0.001, respectively). The areas under the ROC curve (AUC) were as follows: CAR=0.768 (95% CI, 0.705-0.832), white blood cell count (WBC)=0.735 (95% CI, 0.667-0.802), monocytes=0.622 (95% CI, 0.544-0.699), red cell distribution width-standard deviation (RDW-SD)=0.600 (95% CI, 0.522-0.678), SII=0.674 (95% CI, 0.599-0.748), and neutrophil-to-lymphocyte ratio (NLR)=0.687 (95% CI, 0.613-0.761).
Conclusion: CAR and SII could be useful biomarkers to differentiate between ACI and BPPV in vertigo patients, but further validation is needed in larger studies.
Dizziness and vertigo are symptoms associated with disturbances in spatial orientation and movement perception. Vertigo is characterized by various symptoms such as a sensation of rotational movement or a feeling of imbalance.1,2 Vertigo can originate from central sources (affecting the brainstem or cerebellum) or peripheral sources (impacting the inner ear or vestibular nerve). Peripheral vertigo is more commonly observed than central vertigo.3 Additionally, benign paroxysmal positional vertigo (BPPV), which is one of the causes of peripheral vertigo, is the most prevalent among peripheral etiologies.4 Acute cerebellar infarction (ACI) is a frequent cause of central vertigo.5 Central causes of vertigo, such as cerebrovascular disease, can lead to serious consequences if not accurately diagnosed.2 Therefore, distinguishing between ACI and BPPV is of paramount importance. However, this differentiation may not always be possible solely based on clinical examination findings.
In patients presenting with complaints of dizziness, various investigations and cranial imaging studies may be required to clarify the diagnosis. This process is time consuming and may create an economic burden.2,6 Previous studies have shown that approximately 3.3% of emergency department visits are related to dizziness and vertigo, with around 3-5% of these resulting in stroke.7,8 Cerebellar infarction typically accounts for approximately 2.3% of acute strokes, often resulting from occlusion of the posterior inferior cerebellar artery (PICA), superior cerebellar artery (SCA), or anterior inferior cerebellar artery (AICA).5 Specifically, the medial branch of the PICA supplies the posterior inferior region of the cerebellum. This region regulates critical functions such as balance, coordination, and movement control.9
Diffusion-weighted magnetic resonance imaging (MRI) is commonly used to detect early cerebral infarction. However, diffusion-weighted MRI in early-stage vertigo can sometimes yield negative results, leading to diagnostic errors in identifying central causes. Specifically, vertebrobasilar strokes are prone to showing a false-negative pattern on diffusion-weighted MRI, likely due to small lesions and magnetic susceptibility artifacts.9 Due to its high cost, access to MRI may not always be available.10
In patients presenting to outpatient clinics with dizziness, neuro-ophthalmological tests such as gaze-evoked nystagmus, skew deviation, and the head-thrust test may be useful in distinguishing between ACI and BPPV. However, these tests often lack sufficient sensitivity and specificity in the emergency department. Therefore, additional investigations are needed to distinguish between ACI and BPPV.11,12
Systemic immune-inflammatory index (SII) is an inflammatory biomarker calculated based on the peripheral blood counts of lymphocytes, platelets, and neutrophils, and it has been suggested to be associated with the prognosis of various diseases in recent years.13 Additionally, C-reactive protein-to-albumin ratio (CAR) is also used as a marker indicating systemic inflammation.14 This study was designed based on the hypothesis that CAR and SII may be useful parameters for distinguishing between BPPV and ACI due to PICA occlusion in patients presenting with vertigo.
Methods
This study was conducted retrospectively on 2545 patients who presented to our hospital with complaints of dizziness, imbalance, and nausea between 2017 and 2024. Among these patients, 100 had a diagnosis of ACI due to PICA occlusion, and 102 were diagnosed with BPPV. Exclusion criteria for the study included patients presenting with typical findings such as Wallenberg Syndrome, which specifically affects the brainstem clinically, in addition to walking ataxia and horizontal nystagmus, sudden hearing loss, pediatric patients under 18 years of age, those with chronic hepatitis or liver cirrhosis, severe otitis, upper respiratory tract infection symptoms, vestibular neuritis, and patients with oncological or hematological diseases. The inclusion criteria for our study involved patients with a definitive diagnosis, especially those with medial branch PICA occlusion overlapping clinically with BPPV, confirmed by magnetic resonance angiography, diffusion-weighted MRI, and/or computed tomography angiography. The BPPV patient group also included patients with normal brain MRI and CT imaging, supported by bedside diagnostic maneuvers such as Dix-Hallpike and Pagnini-McClure.15
The blood parameters of the patients were analyzed using an autoanalyzer (Sysmex XN1000 hematology analyzer, Kobe, Japan) available in our hospital’s biochemistry and hematology laboratory. The blood parameters of patients at the time of initial hospital admission, including C-reactive protein (CRP), albumin, lymphocyte count, platelet count (PLT), and neutrophil count, eosinophil count, basophil count, red blood cell count (RBC), hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), white blood cell count (WBC), and mean platelet volume (MPV), along with neutrophil-to-lymphocyte ratio (NLR), SII, CAR, demographic data, and comorbidities were recorded for statistical analysis.
The CAR was determined by dividing the C-reactive protein level by the albumin level. The SII was derived by multiplying the platelet count with the neutrophil count and then dividing by the lymphocyte count (SII=platelet count × neutrophil count / lymphocyte count).16
This research adhered to the ethical principles outlined in the Declaration of Helsinki. The study received approval from the Aksaray University Clinical Research Ethics Committee on 07.12.2023 with the decision number 2023/23-15.
Statistical analysis
The results of the study were reported as median (min-max). The data distribution pattern was assessed using the Kolmogorov-Smirnov test (p<0.05). The Mann-Whitney U test was used to compare groups for data that did not follow a normal distribution. Binary outcomes were compared using the Chi-square or Fisher’s exact test. The ROC curve analysis was conducted to evaluate the areas under the curves of various blood analysis parameters. All statistical analyses were performed using SPSS software for Windows, version 23.0 (SPSS Inc., Chicago, IL). A p-value less than 0.05 was considered statistically significant. Exact p-values and confidence intervals were used. Standard guidelines were followed in reporting the results of clinical trials and studies assessing diagnostic tests.
Results
In total, 202 patients with vertiginous symptoms were included in the study. The BPPV group comprised 102 patients (67 females/35 males, mean age: 45±19 years) and the ACI group comprised 100 patients (59 females/41 males, mean age: 68±14 years). The groups were gender matched (p=0.327, X2=0.962), however, the ACI group was significantly older compared to the BPPV group (p<0.001).
According to the Mann Whitney comparison, the median eosinophil, basophil, RBC, hemoglobin, hematocrit, RDW-CV, MCV, platelet and MPV values did not significantly differ between the groups (p=0.113, p=0.439, p=0.333, p=0.423, p=0.241, p=0.058, p=0.808, p=0.406 and p=0.691, respectively). On the other hand, the median CRP (normal range: 0-5 mg/L), CAR (not standardized, but typically low in healthy individuals), WBC (normal range: 4-10 × 109/L), neutrophil (normal range: 2-7×109/L), monocyte (normal range: 0.12-1.2×109/L), RDW-SD (normal range: 35-56 fL), SII (not standardized) and NLR (median=1.65, range=1.2-2.15)17 values were significantly higher (p<0.001, p=0.003, and p=0.014, respectively), and the mean albumin (normal range: 35-52 g/L) and lymphocyte (normal range: 0.80-4 × 109/L) values were significantly lower in the ACI group, compared with the BPPV group (p<0.001 and p=0.019, respectively) (Table 1).
- Comparison of the blood analysis parameters between the groups.
Table 2 presents the comparison of the frequencies of various comorbid conditions between the groups. The frequency of epilepsy, migraine, asthma, COPD, DM, and coronary artery disease did not significantly differ between the groups (p=0.721, p=0.369, p=0.127, p=0.98, p=0.121 and p=0.107, respectively). Conversely, the frequency of arterial hypertension, hyperlipidemia, and congestive heart failure was significantly higher in the ACI group compared to the BPPV group (p<0.001).
- Comparison of the frequencies of various comorbid conditions between the groups.
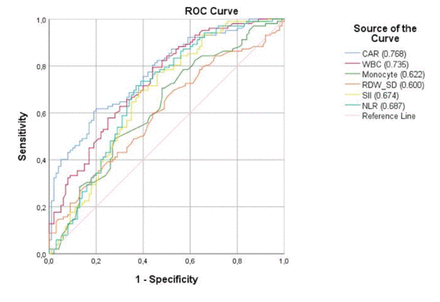
Figure 1 demonstrates the ROC curve of numerous blood analysis parameters in association with ACI. The areas under the curve (AUC) were as follows: The value for CAR is 0.768 (95% CI, 0.705-0.832). The value for WBC is 0.735 (95% CI, 0.667-0.802). The value for Monocyte is 0.622 (95% CI, 0.544-0.699). The value for RDW-SD is 0.600 (95% CI, 0.522-0.678). The value for SII is 0.674 (95% CI, 0.599-0.748). The value for NLR is 0.687 (95% CI, 0.613-0.761).
- Demonstrates the ROC curve of numerous blood analysis parameters in association with acute cerebellar infarct. CAR - C-reactive protein-to-albumin ratio; WBC - white blood cell; RDW_SD- red cell distribution - standard deviation; SII - systemic immune-inflammatory index; NLR - neutrophil-to-lymphocyte ratio
Discussion
The results obtained in the present study showed that CAR and SII values were significantly higher in the ACI due to PICA occlusion group compared to the BPPV group. Furthermore, ROC curve analysis demonstrated that CAR, SII, monocyte, RDW-SD, NLR, and WBC values were predictive of ACI.
Lesions in the vestibular portion of the cerebellum typically lead to clinical manifestations similar to BPPV; in such cases, patients present with symptoms such as a sensation of spinning, ataxia, and nausea.9 Types of ataxia associated with lesions in the vestibular cerebellum are typically cerebellar, vestibular, and sensory ataxias.9 These ataxias are characterized by symptoms such as loss of coordination, balance disturbance, and irregularities in eye movements. Cerebellar ataxia affects the accuracy and timing of movements, while vestibular ataxia disrupts balance and body posture, and sensory ataxia arises from the loss of proprioceptive sensation. These symptoms particularly manifest as a result of ischemic events in regions supplied by the medial branch of PICA, reflecting dysfunction of the vestibular cerebellum.9 In lesions affecting other parts of the cerebellum, symptoms such as dysarthria, dysmetria, dysdiadochokinesia, and ataxia are observed.5 Therefore, focal neurological examination findings may not be present in all patients with ACI, and these patients may be misdiagnosed as BPPV. In fact, approximately 10% of patients with ACI may present with isolated vertigo, meaning they may present with vertigo without localized findings on examination of sensory, motor, reflex, cranial nerve, or extremity coordination.5 Most of these cases are infarctions of the medial branch of the PICA (96%).5,9,18 In summary, these two different clinical presentations can overlap and be confused with each other. In patients with ACI, the misdiagnosis rate is estimated to be 35%, and patients with missed diagnosis of cerebellar infarction are generally at a higher risk of complications, with mortality rates reaching up to 40%.8,19 In addition to imaging techniques, other easily accessible and cost-effective diagnostic parameters such as biomarkers are needed to distinguish between these 2 different clinical presentations. In the present study, higher levels of SII and CAR were found in patients with ACI due to PICA occlusion compared to BPPV. In the early stages of ischemic stroke, there is an increase in acute phase reactants and neutrophil levels, accompanied by a decrease in lymphocyte counts.5 Since ACI is also an ischemic stroke, it is expected that there will be an increase in these parameters. However, BPPV is a condition caused by an otolith in the semicircular canals of the inner ear. This condition most commonly occurs in the posterior semicircular canal. In other words, BPPV is a mechanical event rather than inflammation.5 Both conditions may have similar clinical manifestations.18 Therefore, we believe that in patients presenting with acute dizziness without focal neurological findings, MRI along with these 2 systemic inflammation parameters could be used to differentiate between BPPV and ACI due to PICA occlusion.
In acute ischemic stroke (AIS), neutrophils are the initial responders to the brain area affected by ischemic injury. The quantity of these cells in the bloodstream is directly linked to the extent of cerebral infarction, suggesting that neutrophils play a crucial role in the pathophysiology of AIS. Additionally, an inflammatory response is known to occur in all phases of AIS, and microglia along with astrocytes become activated soon after the ischemic event in the brain. This activation triggers the release of pro-inflammatory cytokines and chemokines, thereby initiating the inflammatory process. This process is one of the primary mechanisms of secondary brain damage, underscoring the significant role of this condition in the pathophysiology of AIS.20 The human immune system is divided into 2 parts: innate and adaptive immune systems. In ischemic strokes, neutrophils migrate to the intraparenchymal perivascular space 6 to 24 hours after ischemia and damage the blood-brain barrier with the cytokines they release into this space.16,20 This process is part of the pathophysiological mechanism of AIS. Furthermore, lymphocytes migrate into the area 3 to 6 days after stroke and play a regulatory role by inducing neuroprotection. SII is a parameter reflecting systemic inflammation. It is stated that SII is a potential marker for both diagnostic assessment and predicting early hospital mortality in stroke cases.16 In the present study, patients with ACI due to PICA occlusion had higher SII levels compared to patients with BPPV.
The CAR reflects systemic inflammation and is obtained by dividing CRP levels by albumin levels.14 As far as we know, CAR has not been investigated in patients with central and peripheral vertigo. However, in a study focusing on CRP, one of the parameters of CAR, no significant difference was observed in CRP levels between the 2 patient groups.21 In another study,22 which compared patients with peripheral vertigo to a healthy control group, no difference was detected in CRP levels between the 2 groups. The likely reason for this could be the absence of inflammation in the pathophysiology of BPPV, which is a disease caused by an otolith. However, it is known that inflammation occurs in ischemic stroke conditions such as ACI, which in turn causes neuronal damage.20 In the present study, it was found that the CAR level, which was calculated taking into account albumin, was statistically significantly higher in patients with ACI due to PICA infarction, one of the causes of central vertigo, compared to patients with BPPV, one of the causes of peripheral vertigo. In the light of all these, we believe that CAR, one of the indicators of inflammation, may be a useful parameter for differentiating ACI due to PICA occlusion from BPPV.
The limitations of this study include potential recording errors inherent in retrospective studies, biases in patient selection, single-center data source, a relatively small sample size, and unforeseen natural variability in blood parameters, especially in patients with ACI who are in an older age group and have comorbid conditions such as hypertension, congestive heart failure, and hyperlipidemia. Additionally, the study did not include long-term follow-up data for patients. These limitations may hinder definitive conclusions regarding the applicability of findings to the general population and the diagnostic value of biomarkers. Therefore, future studies addressing these limitations can further clarify the role of CAR and SII in the management of vertigo.
Conclusion
The results obtained in the present study demonstrated that CAR and SII could be potential biomarkers in distinguishing between ACI due to PICA occlusion and BPPV. These findings could significantly aid in the development of rapid and cost-effective diagnostic tests in emergency departments. We believe that CAR and SII cannot replace more advanced diagnostic methods such as MRI, but they can be used as an adjunctive tool for diagnosis in situations where these advanced diagnostic methods are not available. Before the results of this study are integrated into clinical practice, they need to be confirmed in larger patient populations and different clinical settings.
Future studies could further clarify the diagnostic value of these biomarkers and strengthen their role in the management of vertigo.
Acknowledgement
We would like to thank Enago, a member of Crimson Interactive Pvt. Ltd. for their assistance in language editing.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received August 4, 2024.
- Accepted October 17, 2024.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.