Abstract
Weight loss has substantial health benefits, but it is not risk-free. Various neurological disorders have been reported following bariatric surgery-induced weight loss. Here, we report 3 patients who developed multiple sclerosis (MS), one of whom also developed myasthenia gravis (MG), shortly after significant weight loss. Two patients lost weight by following a diet plan and one underwent bariatric surgery. There may be an association between significant weight loss and the development of an autoimmune neurological disorder such as MS or MG; a high index of suspicion is required.
Obesity is a major worldwide health problem and is associated with increased morbidity and mortality. Common weight loss strategies include diet, exercise, and bariatric surgery. Weight loss has health, social, and economic benefits. However, several neurological disorders have been reported following bariatric surgery-induced weight loss.1 Fad dieting is also a potential health problem for those who try to achieve short-term weight loss. However, the mechanism by which weight loss, whether induced by bariatric surgery or other methods, triggers immune-mediated neurological disorders is largely unknown. In a recent case series, Bitarafan et al2 reported 4 patients who developed multiple sclerosis (MS) following weight loss. We herein report 3 patients who developed MS, one of whom also developed myasthenia gravis (MG), following significant weight loss.
Case Report
Patient information. Patient 1. An 18-year-old male who presented with fatigable ptosis, diplopia, dysarthria, and proximal limb weakness after following a low-calorie diet for 2 months (Table 1). He lost 58 kilograms (kg) of body weight, and his body mass index (BMI) decreased from 35.5 to 16.8 in 3 months. He was diagnosed with generalized seronegative MG based on clinical presentation and single-fiber electromyography findings. The symptoms of MG resolved completely with pyridostigmine, prednisone, and azathioprine. He subsequently noted bilateral lower limb numbness 5 months after weight loss.
Timeline table shows demography, clinical features, investigations, therapeutic intervention and outcome in 3 patients with MS developed after weight loss.
Patient 2
A 28-year-old male who presented with abnormal eye movement, paresthesia in upper and lower limbs, and imbalance (Table 1). He underwent a sleeve gastrectomy 18 months prior to presentation, followed by a low-carbohydrate high-protein diet, and lost 87 kg. His BMI decreased from 55 to 26 in 6 months. His past history was significant for an episode of upper and lower limb paresthesia and sense of heaviness that occurred 10 months after surgery. The past episode was attributed to discontinuing multivitamins, and resolved shortly after reinitiating oral vitamin B complex supplement.
Patient 3 is a 23-year-old male presented with diplopia and right arm and leg paresthesia (Table 1). He reported bilateral lower limb paresthesia extending to the level of the umbilicus 2 years prior. At that time, he intentionally lost 50 kg by following the Atkins diet. His BMI dropped from 49 to 33.5 in 6 months.
Clinical findings
Patient 1 had a normal neurological examination at the time of onset of lower limb numbness. Patient 2 had gaze-evoked nystagmus, appendicular ataxia, and impaired tandem gait (Table 1). Patient 3 had left abducens nerve palsy, right hemisensory loss of pain and temperature, right upper limb dysmetria, and impaired tandem gait.
Diagnostic assessment
All patients had extensive laboratory tests including routine blood workup, vasculitis screening, thyroid function test, hepatitis B, C and human immunodeficiency virus (HIV) tests, vitamin E, B1, B6, B12, folate, copper and zinc levels. Patient 1 had a high antinuclear antibody (ANA) titer of 1:1,280 and a low vitamin D level (Table 1). Patient 2 had a low vitamin D level. Cerebrospinal fluid was normal. Patient 3 had normal laboratory tests.
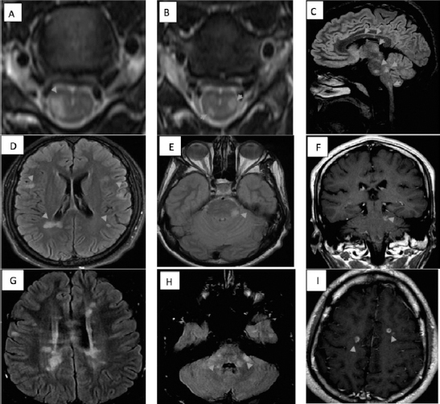
All patients had brain and spinal cord magnetic resonance imaging (MRI) (Figure 1, Table 1). Patient 1 had multiple demyelinating lesions, a few of which were enhancing, located at subcortical and juxtacortical areas, corpus callosum, brainstem, and spinal cord, fulfilling the 2010 McDonald MRI criteria for dissemination in space and time (Figure 1A, B and C).3 Patient 2 had multiple supra- and infra-tentorial non-enhancing demyelinating lesions fulfilling MRI criteria for dissemination in space (Figure 1D, and E).3 Repeat MRI showed new enhancing lesions in the brainstem, fulfilling MRI criteria for dissemination in time (Figure 1F).3 Patient 3 had extensive white matter lesions, a few of which were enhancing, fulfilling the MRI criteria for dissemination in time and space (Figure 1G, H and I). 3
Magnetic resonance imaging A&B) Cervical T2-weighted axial image from patient 1 showing hyperintense signals in the lateral cord. C) Sagittal fluid attenuation inversion recovery (FLAIR) image from patient 1 showing multiple hyperintense signals in supra-and infratentorial areas. D&E) Axial FLAIR image from patient 2 showing multiple hyperintense signals in supra- and infratentorial areas. F) Coronal contrast enhanced sequence from patient 2 showing a contrast-enhanced lesion. G&H) Axial FLAIR image from patient 3 showing multiple hyperintense signals in supra- and infratentorial areas. I) Axial sequence from patient 3 showing contrast-enhanced ring and open-ring lesions.
Therapeutic intervention
All patients received pulse methylprednisolone for the acute relapse (Table 1). Patient 1 elected to continue azathioprine and prednisone that had been initiated for his MG. Patient 2 received natalizumab. Patient 3 received subcutaneous b1a interferon injections.
Follow-up and outcomes
All patients had no clinical or radiological relapses at one-year follow-up (Table 1).
Discussion
We reported the development of MS after weight loss in 3 patients, one of whom also developed MG. Two patients lost weight by following a diet and the third underwent sleeve surgery. All patients had significant weight loss (50-87 kg) in 3-6 months. Serum levels of vitamins and trace elements were normal in all patients, except for a low vitamin D level in patients 1 and 2. All patients had no clinical or radiological relapses at one-year follow-up while on disease-modifying therapy (Figure 1, Table 1).
Obesity early in life is associated with a higher risk of MS.4 One postulated mechanism is that obesity in childhood and adolescence is associated with vitamin D deficiency.5 Furthermore, obesity is associated with chronic low-grade inflammation and increased production of proinflammatory adipocytokines.6 Leptin is an adipocyte-derived hormone that promotes a proinflammatory immune response and constrains anti-inflammatory regulatory T-cell activities, whereas ghrelin, a gastrointestinal peptide hormone that acts centrally to stimulate food intake, has an anti-inflammatory effect, and was shown to attenuate experimental allergic encephalomyelitis in mice.7,8 It has been reported that leptin level decreases and ghrelin level increases after bariatric surgery-induced weight loss.9 Thus, one would expect a protective effect of weight loss against an autoimmune disease. Nonetheless, the occurrence of an autoimmune disease after significant weight loss has been reported in 4 other patients: 2 women developed systemic lupus erythematosus and 2 men developed rheumatoid arthritis.10 In a recent case series, Bitarafan et al2 hypothesized that nutritional deficiencies and inflammatory cytokines release have a role in the development of MS after weight loss.
An increase in post-bariatric surgery total ghrelin level has been demonstrated. Despite a supposed anti-inflammatory role, the contribution of the active (acylated) and inactive (des-acylated) isoforms were not measured; therefore, it is possible that the total ghrelin level was influenced by the inactive isoform.9 In addition, it is not yet known whether the immune modulatory effect of glucagon-like peptide-1 and cholecystokinin has a role in the pathogenesis of MS after weight loss. It is possible that immune dysregulation occurs as a result of abrupt disturbance in the ratio between these hormones/peptides, rather than changes in their serum levels, or due to alternation in the gastrointestinal microbiome after weight loss/bariatric surgeries.
In conclusion, the occurrence of neurological autoimmune diseases after weight loss may have been coincidental or triggered by environmental factors in the presence of genetic susceptibility. As it remains uncertain whether gastrointestinal hormones have a role in the development of autoimmune disease, further research is required. Nonetheless, a high index of suspicion in such cases is warranted to enable timely diagnosis.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received July 24, 2017.
- Accepted January 3, 2018.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.