ABSTRACT
Marchiafava-Bignami disease (MBD) is a rare neurological disorder typically occurring in alcoholic patients. The main disease mechanism is hypothesized to be vitamin B-complex deficiency due to malnutrition. In the literature, there have been few reported cases of the disease occurring in patients who have undergone bariatric surgery. This paper presents a case study of a 45-year-old non-alcoholic female who underwent a laparoscopic sleeve gastrectomy presenting with progressive worsening weakness of her lower limbs and slurred speech. Her condition continued to deteriorate, and she became mute and bedbound. The MRI revealed distinctive characteristics consistent with MBD. The diagnosis was confirmed following the exclusion of all other possible diagnoses. She was treated with multivitamins and had a significant improvement. Additionally, we conduct a review of similar cases of this condition occurring after bariatric surgery. This report sheds light on the occurrence of this uncommon condition after the bariatric procedures.
Marchiafava-Bignami disease (MBD) is a rare neurological disorder that affects the corpus callosum, causing demyelination and necrosis. It was first described in association with alcohol intake.1 The etiology of MBD is thought to be either toxic or nutritional, but the exact etiology is complex and not completely understood. The main pathophysiological mechanisms of the disease include cytotoxic edema, the breakdown of the blood-brain barrier, demyelination, and necrosis. Moreover, it is believed that vitamin B-complex deficiency plays a significant role in the pathophysiology.2
The presentation of MBD varies widely, making it difficult to diagnose. Typically, the condition is diagnosed using magnetic resonance imaging (MRI) and is treated with vitamin supplementation, particularly thiamine.3 Here, we present a case of MBD following sleeve gastrectomy and emphasize the need to consider the condition in patients presenting with neurological symptoms after bariatric surgery.
Case Report
Patient information
A 45-year-old female who had undergone laparoscopic sleeve gastrectomy 40 days prior to her presentation
Clinical findings
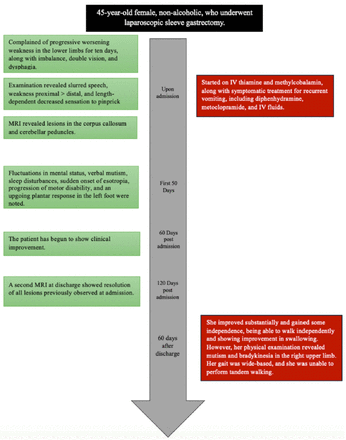
The patient presented to the emergency department (ED) after experiencing gradual-onset progressive lower limb weakness. Ever since her surgery, she had experienced recurrent daily vomiting and abdominal discomfort, which led to her being unable to tolerate oral intake. She had visited the emergency department 3 times for these symptoms and had been treated supportively with IV hydration and antiemetics, reassured, and discharged home. She did not take any vitamin supplements post-surgery, prompting their prescription after one of her visits to the ED. She presented to our hospital after experiencing a gradual onset of progressive bilateral lower limb weakness over the 10 days prior to her presentation. Upon admission, she was bedbound due to her extreme weakness, requiring assistance with all of her activities of daily living (ADLs), including feeding. Her weakness was associated with imbalance and an increased tendency to fall. Furthermore, the patient complained of double vision, particularly with near vision (when attempting to read a book) and dysphagia. There were no other neurological deficits, and her review of the systems was unremarkable. Examination upon admission showed normal vital signs. She was conscious, alert, and oriented to time, person, and place. Her language and memory examinations were normal. However, she looked pale and lethargic. While her cranial nerve examination was unremarkable, she had slurred speech. Furthermore, her motor examination revealed giveaway weakness in both her upper and lower limbs. This weakness was more prominent in the proximal muscles. Examination of the deep tendon reflexes (DTR) was +2 in the upper and lower limbs bilaterally, with downgoing plantars and no pathological reflexes seen. The patient’s sensory examination was intact in the upper limbs. In the right lower limb, however, there was a length-dependent decreased sensation to pinprick up to the knee. Vibration, temperature, and proprioception were intact bilaterally in both lower limbs. Her cerebellar examination was unremarkable in both the upper and lower limbs. She was unable to walk, so her gait was not assessed.
Diagnostic assessment
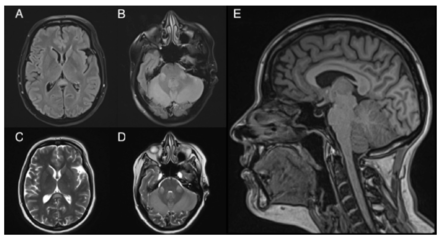
The patient’s initial blood work was positive for electrolyte imbalance, including hypernatremia (171.32–186 mmol/L), with mildly elevated Liver Function Tests (LFTs) (AST 150–160 unit/L, ALT 214.2–336 unit/L) and an acute kidney injury (AKI), which was attributed to volume loss from the patient’s recurrent vomiting. The rest of the blood work showed the following: 13.7 nmol/L folate, 163.25 nmol/L thiamine, 115.58 nmol/L B6, and 417 pmol/L B12, all of which were normal. Hepatitis B, hepatitis C, and HIV screenings were negative. A cerebrospinal fluid (CSF) study was performed and showed high glucose (4.37 mmol/L) and high protein (0.65 g/l) with no RBCs or WBCs. Nerve conduction studies (NCS) performed on both upper and lower limbs were normal. A paraneoplastic panel and vasculitis markers were sent, and the results of both were negative. Initial MRI (Figure 1) demonstrated multiple scattered T2WI/FLAIR high-signal intensity lesions in the cerebellar peduncles and the splenium of the corpus callosum. Computerized tomography of the chest, abdomen, and pelvis was done as part of a paraneoplastic workup and only revealed a benign hepatic lesion and a thyroid mass, which was investigated by the endocrinology team and thought to be benign.
- An axial FLAIR image illustrates A-B) 2 hyperintense lesions in the cerebellar peduncles along with a lesion in the splenium of the corpus callosum. C-D) illustrate an axial T2 image of the same lesions, and E) demonstrates a sagittal T1 image which shows the clear involvement of the splenium along with a generalized atrophy of the corpus callosum. These MRI are consistent with Marchiafava-Bignami disease (MBD).
Therapeutic intervention
The patient was promptly started on IV thiamine (250 mg IV for 3 days followed by 100 mg daily indefinitely) and methylcobalamin (500 mcg IV/IM 3 times weekly for 3 months, and then 500 mcg IV/IM every one to 3 months), along with symptomatic treatment for the recurrent vomiting with diphenhydramine, metoclopramide, and IV fluids.
Follow-up and outcomes
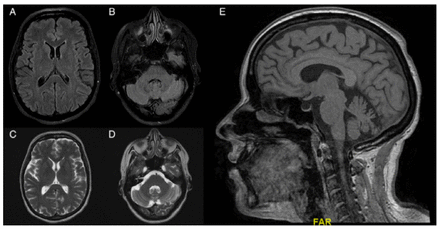
Regarding her clinical course, the patient’s mental status deteriorated throughout her first 50 days of admission, with worsening drowsiness and fluctuation in her orientation to time, person, and place. Additionally, she lost the ability to follow complex commands. One month after her admission, the patient developed verbal mutism and only communicated via hand gestures. She also developed sleep disturbances consisting of hyposomnia. Furthermore, she developed sudden-onset esotropia, which was overcome with an oculocephalic maneuver. Additionally, her motor disability progressed. Worsening weakness was seen in her limb, with no movement against gravity. Moreover, the patient became hyperreflexic in both her upper and lower limbs and developed a non-sustained clonus in the right foot, an upgoing plantar response in the left foot, and a withdrawing plantar response in the right foot. As a side effect of metoclopramide, she developed abnormal hyperkinetic movements in her jaw. These abnormal movements were attributed to metoclopramide, given the temporal relationship with the drug administration. The patient started to show clinical improvement 2 months after her presentation. A second MRI upon discharge (Figure 2) showed the resolution of all lesions that were previously seen upon admission. The patient’s total length of hospital stay was 4 months.
- Illustrates the resolution of the identified lesions upon presentation.
- Illustrates the timeline of our patient’s presentation.
At follow-up (2 months after discharge), the patient had improved substantially and gained some independence in her ADLs, particularly in relation to feeding and dressing. She was able to walk independently, and her swallowing had improved. However, her physical examination showed a masked face, mutism, and bradykinesia in the right upper limb, while all previous pathological reflexes resolved. Her gait was wide-based, and she was not able to tandem.
Discussion
The MBD has been classically described in alcoholics, specifically wine drinkers.1 Multiple MBD cases with numerous different causes have been reported. The main existing hypothesis is that the mechanism of MBD is associated with vitamin B-complex deficiency. It is characterized by morphologic and metabolic changes due to demyelination and micronecrosis of the corpus callosum that vary throughout the presentation of the illness. It has also been suggested that an inflammatory reaction may accompany demyelination and micronecrosis.2 The clinical manifestation of the condition lacks specificity, as it can manifest acutely, subacutely, or chronically. Moreover, the initial symptoms are vague, as the patient’s first presentation may include a range of symptoms, from weakness to alterations in behavior.4 As a result, diagnosing the condition can be difficult for non-alcoholics, particularly in the absence of a clear preceding event. Rare etiologies have been implicated in the development of MBD, including anorexia nervosa, diabetes, and hepatitis C.5 Almost all cases described in the literature have one thing in common: malnutrition. While bariatric surgery may be necessary for the treatment of morbid obesity, it is important to recognize its potential drawbacks. In addition to surgical morbidities such as bowel leakage or bleeding, it can also lead to nutritional deficiencies, including those of water-soluble vitamins, which may contribute to certain neurological manifestations.6 Another documented case of non-alcoholic MBD highlighted the occurrence of the condition following sudden changes in serum osmolality.7 This is similar to the case of our patient, whose recurrent vomiting and reduced oral intake post-surgery led to hypernatremia and AKI.
Based on the clinical presentation characterized by generalized weakness and asymmetric sensory deficits, our provisional diagnosis initially favored nutritional neuropathy. Subsequent development of central signs, including confusion and disorientation, along with the patient’s initial presentation of double vision and ataxia, raised suspicion for Wernicke’s encephalopathy, prompting an MRI investigation. The MRI findings illustrated in Figure 1, along with the clinical presentation of confusion, disorientation, memory impairment, and weakness, made the diagnosis of MBD more likely. While the diagnosis changed, our treatment approach remained unchanged, as the patient demonstrated improvement with vitamin replacement and supportive care.
We selected 3 different cases of MBD post-bariatric surgery reported in the literature. These are summarized in Table 1. Interestingly, all patients were female, with a mean age of 52.25 years. Each reported case underwent a different type of bariatric surgery, indicating that the mechanism of the disease is related to malnutrition rather than the type of surgery itself. Yıldırım Z et al8 reported a patient who presented with nausea, vomiting, and delusion seven days after gastric balloon surgery, while Bachar M et al9 described a patient who presented with weakness 23 years after gastric bypass surgery. Lastly, Salazar G et al10 reported a case that presented with generalized weakness, forgetfulness, apraxia, and alien limb syndrome five years after bariatric surgery. Even though the presenting symptoms differed from one case to another, all of these cases involved a preceding bariatric surgery as the main cause of malnutrition, emphasizing the importance of vitamin supplementation after bariatric surgery and adding this surgery to the list of potential causes of MBD. Noteworthy, the splenium part of the corpus callosum was specifically implicated in 2 out of 3 cases, while Salazar G et al’s case demonstrated a lesion involving the entire corpus along with generalized atrophy. Interestingly, our patient exhibited a delayed response to treatment despite the rapid initiation of thiamine. This delay may be attributed to an accompanying oxidative stress and micronecrosis that can occur during the demyelination process caused by thiamine deficiency.2 Additionally, reported cases in the literature have demonstrated varying responses, ranging from spontaneous recovery to no response to thiamine, highlighting the complex nature of the pathophysiology, management, and natural history of MBD.5 Our patient also had a prolonged hospital and recovery course, showing gradual improvement over a six-month period following her initial presentation. Despite not fully returning to her baseline, she showed significant clinical improvement. On the other hand, Yıldırım Z et al and Bachar M et al’s cases showed complete recovery 2 weeks after initiating treatment. A potential reason for the poorer prognosis in our case may be attributed to the lesions detected in the MRI. Specifically, our patient exhibited lesions located beyond the splenium of the corpus callosum, as illustrated in Figure 1. It has been previously reported that lesions confined to the splenium of the corpus are associated with better prognoses.5 In addition, the Glasgow Outcome Scale assessment indicates that our patient exhibited moderate disability, a result consistent with patients diagnosed with alcoholic MBD rather than non-alcoholic MBD. Lastly, our case highlights the importance of considering MBD in post-bariatric surgery patients who present with both peripheral and central neurological signs and symptoms, particularly given the rising prevalence of obesity and the growing utilization of bariatric procedures as a treatment modality.
- Cases of Marchiafava-Bignami disease (MBD) post-bariatric surgeries.
Conclusion
In this paper, we present a case of MBD in a patient who had undergone laparoscopic sleeve gastrectomy, emphasizing the importance of considering this rare diagnosis in patients experiencing neurological symptoms following bariatric surgery. This study also compares the current case with previous case studies of patients presenting with MBD after bariatric surgical procedures.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received April 15, 2024.
- Accepted November 19, 2024.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.