Abstract
Absent F wave in the stage of spinal shock has been described in cases of traumatic spinal cord injury. The role of F wave in predicting prognosis after conus medullaris infarct has not been described. We describe herein a middle aged-man with a conus medullaris infarct. Both tibial and peroneal F waves were absent on day 4. The left tibial F wave reappeared in the following study on day 18. All F waves reappeared on day 56 at which time the patient was still wheelchair bound. He regained walking on day 105. We hypothesize that reappearance of initially absent F waves post conus medullaris infarct is a good prognostic sign for the return of ambulation. The applicability of this observation requires further research. We also discuss clinical and diagnostic caveats in this case.
F wave is a late motor response elicited by supramaximal electrical stimulations of motor nerves. Electrical impulses travel antidromically through the motor nerve roots to the anterior horn cells (AHC), backfiring of which sends orthodromic motor impulses that travel distally to be captured by a recording electrode placed over a muscle innervated by the stimulated nerve.1 Minimal F wave latency is 25 - 32 ms in upper extremities and 45 - 56 ms in lower extremities.1 Herein, a possible prognostic role for F waves after conus medullaris infarct is illustrated. Such diagnosis requires a high index of suspicion because spine imaging may be normal in the hyperacute stage.
Case Report
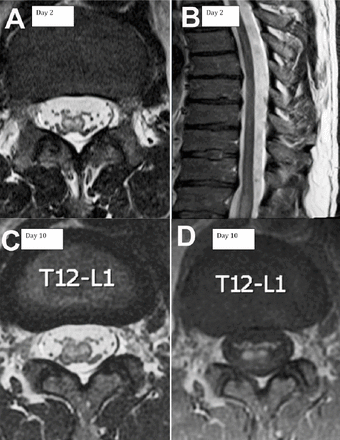
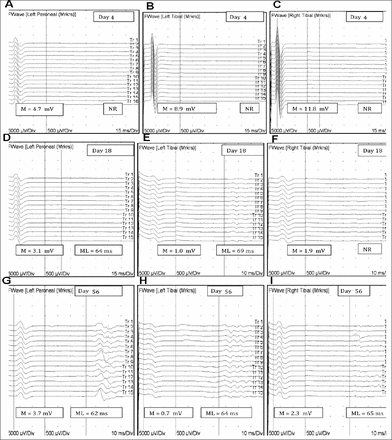
A 48-year-old man presented with acute lower extremity weakness and back pain developed while he was working in a crawl space hyper extending his back. He described feet numbness, urine retention, and constipation. On examination, muscle strength was 4/5 in right iliopsoas and hamstrings, and 0/5 in the remaining muscles of right and all muscles of the left lower limb. Bilateral knee and ankle reflexes were absent. Plantar responses were equivocal. Pinprick sensation was decreased over left leg up to T12 level, and right L3 through S2 dermatomes. Vibration and proprioception sensation were normal. At a peripheral hospital, spine MRI was unremarkable on day 1. Spine MRI on day 2 showed a non-enhancing intra-axial bright T2-weighted signal preferentially involving the anterior part of the cord extending from the conus to T11 (Figure 1a&b). On day 4, NCS showed normal sensory and motor studies except an unrecordable right peroneal motor response (chronic, post ankle fracture). F-waves were not obtainable over the peroneal and tibial nerves (Figure 2a-c). Cerebrospinal fluid protein, glucose, cell count, cytology and flow cytometry were normal. Extensive metabolic, infectious, vasculitis, cardiac, malignancy and thrombophilia workup were unremarkable. He initially received intravenous methylprednisolone and plasma exchange for a presumed transverse myelitis. On day 10, MRI showed smooth enhancement of the ventral nerve roots and conus (Figure 1c&d). The diagnosis was revised to conus medullaris infarct based on the distribution and evolution of MRI findings. Sensory NCS remained normal on day 18. Left peroneal and bilateral tibial compound muscle action potential (CMAP) amplitudes decreased. Left tibial F waves reappeared with a prolonged latency (69 ms). Left peroneal F waves reappeared with poor persistence (6.7%) and prolonged latency (64 ms). Right tibial F waves remained absent (Figure 2d-f). His weakness persisted. Hyperreflexia developed in bilateral knee and left ankle, with left Babinski sign. Right ankle reflex remained absent with equivocal plantar response. On day 56, he demonstrated clinical improvement but was unable to walk. Although CMAP amplitudes remained decreased, all F-waves were obtainable with a slightly prolonged latency and normal persistence (Figure 2g-i). He regained walking with a walker on day 105.
Spine MRI axial (a) and sagittal (b) T2-weighted MRI obtained 2 days after symptoms onset demonstrating T2 hyperintense signal within the conus extending to T11. Axial T2-weighted (c) and contrast enhanced (d) MRI obtained 10 days after symptom onset demonstrating contrast enhancement of the anterior part of the conus and nerve roots.
F-waves (a-c) F responses recorded by stimulating the left peroneal and bilateral tibial nerves at day 4, (d-f) day 18, and (g-i) day 56 after disease onset. M - direct motor response; ML-minimal latency; mV - millivolt, NR - no response; ms - millisecond
Discussion
F-wave is not considered part of the workup in conus medullaris infarct. However, in the absence of upper motor neuron signs, especially with a normal early spine MRI and absent F-wave, confusion about the diagnosis may arise. In a previous case report, F waves were absent in the hyperacute stage (first 4 hours) of anterior spinal cord infarct.2 In our patient, F waves were absent at the time of the first nerve conduction studies (NCS) (day 4) and they may have been absent from the onset. Hiersemenzel et al3 described reappearance of F waves after the stage of spinal shock in 12 patients with a traumatic paraplegia above T10 level, and attributed the early absence of F waves to reduced excitability of motor neurons at the stage of spinal shock. In our patient, the mechanism of injury was ischemia (rather than trauma) and the injury level was at the conus (lumbosacral spinal cord segments) with involvement of the motor nerve roots that showed enhancement on the follow-up MRI. Recovery of left tibial F waves on day 18 coincided with development of hyperreflexia in the same leg. In contrast, at the time of reappearance of right tibial F waves on day 56, right ankle reflex was absent. Thus, we hypothesize that the absent F-waves in the acute stages of conus medullaris infarct might have been due to temporarily unexcitable AHC as a result of spinal shock, spinal cord edema, and possibly oligemia (Figure 1). We would not expect F waves to be recordable in the setting of a diffuse infarction of all AHC in the spinal cord segments subserving the tested nerve.
We are not aware of any reports describing F wave as a prognostic indicator of conus medullaris infarct. We postulate that persistently absent F waves, beyond the stage of spinal shock (2-4 weeks), in conus medullaris infarct indicates severe damage to AHC in the lumbosacral region and poor chance for renervation through collateral sprouting. Conversely, reappearance of F waves may predict motor recovery. In our patient, all F waves had reappeared by day 56, at least 8 weeks before he regained walking. The area of infarct involved the ventral part of the spinal cord supplied by the anterior spinal artery. We suspect the mechanism of infarct was secondary to a hyperextension injury similar to the non-traumatic spinal cord infarct described in novice surfers (surfers myelopathy). In such cases, the postulated mechanisms of ischemia include transient arterial compression during a prolonged back hyperextension in the setting of poor collaterals, vasospasm or thrombosis of the artery of Adamkiewicz, and avulsion of perforating blood vessels.4,5 The prognosis of surfers myelopathy varies from complete recovery to persistent paraparesis.4,5 A normal MRI in the first few days does not rule out spinal cord infarct. In reported case series, the earliest changes (bright T2-weighted signals) appeared after 1-2 days, followed by enhancement appeared within the first week and peaked at 14 -21 days.6,7 Cauda equina enhancement post spinal cord infarct has been described in a few case reports and attributed to a possible disruption of blood-nerve barrier.2,8,9
In conclusion, further studies are required to test our hypothesis that reappearance of tibial and peroneal F waves has a prognostic value in predicting walking in patients with conus medullaris infarct.
Footnotes
Disclosure
The author has no conflict of interest and the work was not supported by or funded by any drug company.
- Received August 27, 2015.
- Accepted March 9, 2016.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.