Abstract
Objective: To compare the efficacy and safety of low-molecular-weight heparin (LMWH) and unfractionated heparin (UFH) in the treatment of patients with cerebral venous sinus thrombosis (CVST), and to provide an appropriate treatment option in these patients.
Method: This is a randomized double blind clinical trial conducted between December 2013 and December 2014. The subjects were selected among patients referred to Neurology Department, Imam Reza Hospital; affiliated to Kermanshah University of Medical Sciences, Kermanshah, Iran. Fifty-two cases of CVST were included in this study and randomly divided into 2 groups. Twenty-six cases received LMWH and the other 26 cases received UFH. The primary outcomes include hospital mortality rate and neurologic deficits as assessed by the National Institutes of Health Stroke Scale (NIHSS). The secondary end point was disability as measured by the Modified Rankin Scale (MRS).
Results: We observed the rate of mortality and neurological deficits and disability based on NIHSS, and the MRS did not differ between the 2 groups.
Conclusion: The efficacy of LMWH and UFH in reduction of neurologic deficit and functional disability in patients with CVST are similar.
Cerebral venous sinus thrombosis (CVST) is an uncommon stroke that, unlike arterial stroke, occurs mostly in adolescent and young children.1,2 The outcome of patients with CVST is variable from complete recovery to persistent neurological damage.3 Regardless of the fact that most patients have a complete or partial recovery, 10% are observe to have persistent neurological damage by 12 months of follow-up.3 According to information from clinical trials and observational studies, anticoagulation is advised as safe and effective treatment for cerebral venous thrombosis.3-5 Anticoagulants can also prevent pulmonary embolism, which makes sinus thrombosis is highly complicated.4-7 However, there is no agreement on what type of heparin to use: low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH).7,8 The advantage of using UFH is that it acts rapidly due to inhibition of IIa factor, and its effects are reversible by administering protamine sulfate; however, binding to plasma proteins, platelet (platelet factor 4), macrophages, and endothelial cells limit its bioavailability and account for the highly variable anticoagulant response and requires dose adjustments based on activated partial-thromboplastin time values.8,9 The clinical benefits of LMWH comprise higher bioavailability more predictable pharmacokinetic profile, dose-dependent plasma levels, a longer half-life, the lower risk of bleeding and immune-mediated thrombocytopenia and heparin-induced osteoporosis.8,9 Because of these clinical advantages, LMWHs have gradually replaced UFH for most indications. Several clinical trials, in patients with deep venous thrombosis and pulmonary embolism indicates that LMWH is as effective as UFH and associated with the fewest hemorrhagic complications.8,10 There are already limited data directly comparing LMWH with UFH in CVST patients. It is critical for the clinician to understand which drugs are most effective and associated with the fewest adverse events. The aim of this study was to compare the efficacy and safety of these 2 types of heparins in the treatment of patients with CVST to provide an appropriate treatment option in such patients.
Methods
Search method
Between 2009 and 2012, we searched MEDLINE, Ovid, CINAHL, and EBSCO for full-text articles written in English with appropriate MESH and free subject terms: 1) CVST; 2)cerebral venous thrombosis; 3) cortical vein thrombosis; 4) intracranial thrombosis. One to 4 aforementioned terms was combined with the terms: treatment, low-molecular-weight heparin, unfractionated heparin.
Study design
This study was conducted as a randomized double blind clinical trial between December 2013 and December 2014. The subjects were selected among patients referred to the Neurology Emergency Room, Imam Reza Hospital; affiliated to Kermanshah University of Medical Sciences, Kermanshah, Iran. The diagnosis of CVST was confirmed by clinical examinations, brain MRI, and magnetic resonance venography (MRV). This trial was registered in the Iranian Registry of Clinical Trials. The local ethics committee approved the protocol of the study. This study was conducted according to the principles of the Helsinki Declaration. A written informed consent was obtained from all patients or their immediate family.
Inclusion criteria
Inclusion criteria were as follows: 1) Diagnosis of CVST confirmed by clinical examinations, MRI, and magnetic resonance venography (MRV), and 2) age >18 old years.
Exclusion criteria
Exclusion criteria were as follows: 1) anticoagulant contraindication, 2) hemorrhagic disorders and thrombocytopenia (platelet count <100000), 3) kidney and liver disease, 4) uncontrolled hypertension (diastolic >110 mm Hg), 4) recent gastrointestinal hemorrhage, 5) papilledema with visual impairment, which necessitate cerebrospinal fluid shunt, and 6) debilitating illnesses other than CVST.
On the basis of a computer-generated random number, eligible patients were assigned into UFH and LMWH groups. The LMWH group received enoxaparin (Alborz Daru, Qazvin, Iran) 1 mg/kg subcutaneously twice a day and the UFH group received heparin (Alborz Daru, Qazvin, Iran) loading-dose of 80 units/kg intravenous (IV) followed by 18 units/kg/hour by continuous IV infusion with dose adjustment to achieve a target activated partial-thromboplastin time (aPTT) of 60-85 seconds. The aPTT was measured at baseline and 6 hours after initiation of the study drug. The results were reported only to an independent and unblinded health care professional. Investigators and patients were blind to the treatments by preprinted medication code labels. The treatment and evaluation were carried out by different subjects. The patients received the doses for 7-10 days followed by oral anticoagulant for at least 6 months depending on the underlying cause (target: international normalized ratio [INR] between 2.5 and 3.5).11
The primary outcomes include hospital mortality rate and neurologic deficits as assessed by the National Institutes of Health Stroke Scale (NIHSS)12 at time of discharge from hospital and after one month. The secondary end point was disability measured by the Modified Rankin Scale (MRS)13 at 30-day follow-up and hemorrhagic complications during hospitalization period. Hemorrhagic complications were classified into: symptomatic intracerebral hemorrhage (sICH) and major extracranial hemorrhage. The sICH was defined as a CT-documented hemorrhage, which was related to exacerbations of initial neurological deficits and/or newly developed neurological deficits after treatment.14
All patients were examined neurologically at baseline by the resident neurologist. Follow-up visits were conducted by the same investigators, and repeated scores on MRS and NIH stroke scale were obtained at time of discharge from hospital after one month. After selecting the subjects their blood sample was taken to measure INR, prothrombin time and activated partial-thromboplastin time, creatinine, blood urea nitrogen, and platelet count. The aPTT was checked 6 hours after bolus or after any rate change. Platelet counts were obtained at baseline and was performed periodically. If the platelet count dropped below 100,000/mm3 or if recurrent thrombosis develops, the heparin product was discontinued. The blood samples were also tested for antinuclear antibody, anti double strain DNA, antiphospholipid antibody, lupus anticoagulant, protein C (prC), protein S (prS), factor V Leiden, hemocystein, and antithrombin III gene mutation.
Statistical analysis
Patient’s demographic and clinical information were recorded in a predesigned checklist. Based on other study,15 we estimated a total sample size of 52 patients considering 5% level of significance and power of 80%. Statistical analysis was performed by the Statistical Package for Social Sciences (version 19.0, SPSS Inc., Chicago, IL, USA). Values were expressed as Means±SD or as percentages. Means were compared by the student’s t-test or one-way analysis of variance (ANOVA) test. The percentage was calculated in the presence and absence group by Pearson’s Chi-square test. The limit of statistical significance was set at p<0.05.
Results
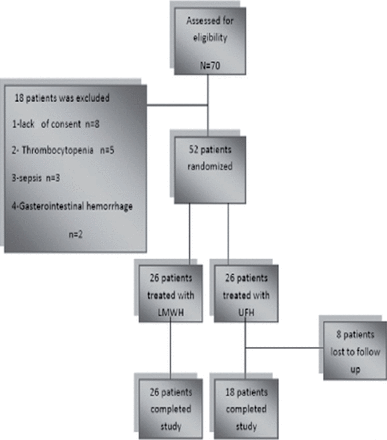
Fifty-two of 70 patients with CVST admitted to the neurology emergency room were randomized. A flow chart of patient enrollment and disposition is provided in Figure 1. There was no significant difference between baseline clinical and demographic characteristics of the different treatment groups (p>0.05) (Table 1).
Consort flowchart. UFH - unfractionated heparin, LMWH - low-molecular-weight heparin
Baseline characteristics of the patient with cerebral venous sinus thrombosis.
The mean sample age was 37.3±15.3 years. Overall, 35 of the subjects were female and 17 subjects were male. Twenty-six patients were treated with LMWH and 26 received UFH. Of the 52 patients who received study medication, 8 subjects (all from the UFH treatment group) were excluded from the study. These patients were considered lost to follow-up, as they transferred to another department or to the care of another physician. They were not used in any of the endpoint analyses.
Twenty-eight female cases had a female-specific risk factor including OCP pill, pregnancy, and postpartum. Although 2 cases were pregnant, they continued their medications without changing it to warfarin. Six cases suffered from thrombophilia including prC, prS deficiency (n=2), factor V Liden mutation (n=2), increased homocystein (n=1), and lupus anticoagulant antibody (n=1). However, in 10 cases no risk factor was found. At end point, mean scores of NIHSS and MRS decreased significantly in the 2 groups (Table 2). There was no statistically significant difference between UHF and LMWH in the mean NIHS and MRS scores during follow-up period. The result for the outcome measures are shown in Table 2.
Outcome of patient with cerebral venous sinus thrombosis.
Two patients died during hospitalization period; one of the patients was in LMWH group and one from UFH group. Furthermore, both patients had hemorrhagic infarcts when hospitalized, and their death was due to increased intracranial pressure and cerebral herniation. During hospitalization period, new seizure was observed in 3 patients. One of them was in LMWH group while the 2 other was from the UFH group (p=0.55). Symptomatic intracerebral hemorrhage was not detected in any patient. Extra cranial hemorrhage including AUB, epistaxis, and hematuria occurred in 3 cases. Two of them were in LMWH group and one of them was in UFH (p=0.99). One patients was treated with UFH and had a deep venous thrombosis in the lower limb while none of the cases in LMWH group identified with similar problem (p=0.40).
Discussion
Low-molecular-weight heparin and UHF have desirable effects on neurological deficit and disability. Patients good outcome cannot easily be attributed to the heparin treatment due to the absence of a control group, and spontaneous recovery cannot be ruled out. Our results are in agreement with data from randomized trials conducted by Misra et al.15 In their study, UFH were administered in 32 and 34 patients in LMWH at 3 months, insignificantly higher number of patients improved completely in LMWH compared with UFH group (30 versus [vs] 20).15 In the prospective cohort study carried out by Coutinho et al,6 there are 119 patients received LMWH and 302 patients received UFH; results revealed after 6 month, the LMWH group had a better outcome (MRS: 0-2). However, the number of patients with complete recovery (MRS: 0-1) has no significant difference between the 2 groups.8
Insignificantly higher number of patients died in UFH (5.6%) compared with the LMWH group (3.8%) (p=0.99). Similarly, Coutinho et al8 observed no significant difference in the mortality rate between the groups LMWH (6%) and UFH (8%) (p=0.7). However, in the study carried out by Misra et al,15 significantly lower mortality rate was seen in the LMWH group (0%) compared with UFH group (18.8%) (p=0.01).15 In the current study, we observed that there were significantly more extracranial hemorrhage in the LMWH treated patients compared with UFH, and the rate of the new sICH has no significant difference between the 2 groups.
These results may be due to the small sample size and lack of evaluation of asymptomatic intracranial hemorrhage rate in our study. Coutinho et al8 observed that the rates of extracranial hemorrhage did not differ between the groups significantly, but the UFH group experienced more intracranial hemorrhage. In contrary to our results, in the study of Misra et al,15 extra cranial hemorrhage was significantly higher in the UFH group. The rate of extracranial thrombotic events in our study and Coutinho et al8 were not different in 2 groups (p=0.40).
Limitations of this study include: being single centric, lack of evaluation of asymptomatic intracranial hemorrhage rate, lack of placebo group, small sample size, and short follow-up period.
In conclusion, this study showed that efficacy of LMWH and UFH in reduction of neurologic deficit and functional disability in patients with CVST are similar. And also the 2 groups showed no significant difference in hemorrhagic and thrombotic events. Although we should remain cautious until our results can be confirmed in a further well designed large.
Footnotes
Disclosure
The authors declare no conflicting interests, support or funding from any drug company.
- Received May 26, 2015.
- Accepted July 29, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.