Abstract
Objectives: To determine physicians’ attitudes and stated practice in the management of patients with spinal muscular atrophy (SMA). We also aimed to explore their knowledge about consensus statement for standard of care in SMA and the role of new treatment modalities in changing the method of practice in the management of these cases.
Methods: This is a quantitative observational cross-sectional study, conducted from February to May 2017 among physicians who manage SMA patients in Kingdom of Saudi Arabia. The study cohort included pediatric neurologists, adult neurologists, and physicians of other sub-specialties who manage SMA patients. We used online and paper-based questionnaires.
Results: Half of the 169 participants were aware of the consensus guidelines for the care of SMA patients. With regard to the newly released Nursinersen treatment protocol for SMA-diagnosed patients, half of the participants were uncertain, and the other half were hesitant about its outcomes. Junior physicians tended to be significantly more inclined to reverse the do-not-resuscitate (DNR) status of an SMA-diagnosed child than more senior physicians.
Conclusion: Our results indicate the existence of wide differences in physician practice with children of SMA disease. Our data demonstrate a need for increased awareness of consensus guidelines and further awareness about the physician’s role in the variability of care for children with SMA.
Spinal muscular atrophy (SMA) is an autosomal recessive disorder. It affects approximately 1 in 10,000 live births, and it occurs in almost 95% of all cases as a homozygous deletion or mutation of the survival motor neuron 1 gene (SMN1).1-5 Spinal muscular atrophy is featured by degeneration of alpha motor neurons in the spinal cord, leading to progressive weakness and muscular atrophy.6-8 It is usually classified into 3 main phenotypes (Type I, II, and III), but an expanded classification system has been used by several experts that includes a fourth phenotype to distinguish adult-onset SMA.8,9 Type I is the most severe form of SMA, representing approximately 60% of SMA cases.8-10
Many questions remain although clinical advancements have been made in the care of children with SMA. Previously, no treatment was available for SMA, and management consisted of supportive measures directed at providing adequate nutrition, respiratory assistance, and treating or preventing complications of weakness. In many aspects of care for these children (nutrition, pulmonary, orthopedic, and rehabilitation medicine), medical decisions are usually based on the clinician’s or institution’s experience or from small studies with low levels of evidence.9
Currently, a new therapeutic option has become available for patients with SMA. Nusinersen is the first approved drug used to treat pediatric and adult patients with SMA; it was approved by the US food and drug administration in late December 2016 after its safety and effectiveness were validated.11 This study aimed to determine pediatrics and adult neurologists’ knowledge and stated practice in the management of children with SMA. This study aimed to investigate the understanding of the knowledge and practices regarding acute and long-term management of children with SMA, especially with the appearance of new modalities of treatment.
Methods
A quantitative observational cross-sectional approach was used to explore physicians’ practice in the acute and long-term management of children with SMA. Online and paper-based questionnaires were sent to all members of the neuroscience forum, a scientific group on smartphones and among physicians attending the 2nd Saudi pediatric neurology society annual conference 2017 (SPNS).12
The study was conducted in the Kingdom of Saudi Arabia from February-May 2017. Our target samples included pediatric and adult neurologists in addition to physicians in other sub-specialties who manage SMA patients. Their compliance with the consensus was measured using a 1-10 Likert scale. The study instrument was subjected to a panel of experts in the field of neurology for the importance of its content to the study objectives and adjustements were made according to feedback from 5 academic experts. The Cronbach’s alpha test of reliability was conducted on the likert-like questionnaire items to assess them for reliability on a piloted sample of 30 subjects.
Data was analyzed using Statistical Package for the Social Sciences, version 21 (IBM Corp., Armonk, NY, USA). A p-value<0.05 was considered significant. Descriptive statistics were used to present means, standard deviations, and percentages. Student’s t-test, z-proportional test, and Chi-squared tests were employed to compare group variables between genders and demographic variables. Multivariate binary logistic regression analysis was employed to explain physicians’ awareness of SMA standards of care and the willingness to change the patients’ code status to DNR in light of impending compromise. The study was approved by the institutional review board and ethical committee of King Saud university, Riyadh, Kingdom of Saudi Arabia (KSU-IRB Number E-17-2307).
Results
The questionnaire items that were measured with likert-like scale were generally reliable, as an evidence with Cronbach’s alpha test of internal consistency equal to (0.87), denoting that the physicians have understood these items consistently and reliably in general. Of 212 physicians, 169 responded to the survey. Approximately half of them (n=87, 51.2%) were male. Pediatric neurologists represented 51.5%; physicians from other specialties were mostly pediatric intensivists (17.8%), pediatricians (13%), or adult neurologists (10.7%). The physicians’ demographics and professional characteristics are shown in Table 1. A total of 49.7% of participants were aware of the international consensus guidelines for management of SMA patients. However, when questioned on a Likert-like rating scale, those who were aware of the consensus (n=73) collectively agreed with 6.6 out of 10 points, denoting that more than 50% generally agreed to the consensus. The mean compliance level was equal to 6.1 out of 10; namely, 61.1% were compliants (Table 2). Regarding characteristics and awareness of the consensus of the SMA patients, the analysis showed that there was a significant association between physicians’ specialty and their awareness of the standards (χ2(4)=18.9, p=0.001). Pediatric neurologists were significantly more aware; however, the pediatric intensivists tended to report their lack of awareness of the consensus on the SMA standards of care. General pediatricians were significantly less inclined to be informed of these standards according to their responses (Table 2). Multivariate logistic regression analysis of the combined and individual associations between physicians’ demographic and professional characteristics with their awareness of the consensus of the standards of SMA patients care showed that the field of physicians’ specialty experience (expertise) was a significant predictor of their awareness with the consensus (OR=0.611; 95% CI=0.480-0.776; p<0.001) (Table 3).
Physician demographic and professional characteristics (N=169).
Physician demographics and bivariate analysis of the associations between professional and working conditions with awareness regarding SMA consensus care protocol.
Multivariate logistic regression analysis of the combined and individual associations between physician demographic and professional characteristics with their awareness of the consensus of the standard of SMA patients care (N=168).
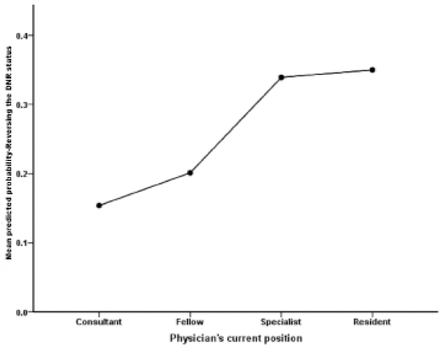
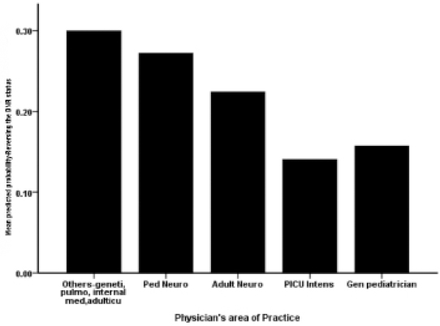
When physicians were asked to select from a list of recent promising treatments for SMA, it was found that only 83 physicians responded and demonstrated awareness of at least one modern modality or more. Two thirds (59.8%) were aware of gene-specific therapy. Another 41% were aware of drug treatments, 6% indicated they were aware of autologous stem cell transplantation as a potential treatment for SMA patients. In response to questions about the newly released medication Nusinersen for SMA patients, half of the physicians (52.1%) were uncertain. Only 34.3% were aware and a few (13.6%) were not aware of the new drug/treatment. Regarding physicians’ belief in the ability of Nusinersen to improve SMA patient outcomes, half (53.3%) were uncertain, and approximately 32% of them believed in the potential of the drug, but the rest (14.8%) did not believe in the potential of this new drug to improve outcomes. Despite the controversy around reversing the code status of the DNR of an SMA patient, physicians were asked to indicate whether they would be inclined to reverse the code status of an end stage SMA patient in light of the promising effects of Nusinersen: Half (52.1%) of them were unsure, another 23.1% of them advised with approval, and a smaller proportion (24.9%) advised with disapproval. They were asked to indicate whether it was acceptable to intubate an SMA patient in light of an impending respiratory compromise: Half of the physicians (50.3%) agreed, 33.7% were uncertain, and several (16%) of them disagreed (Table 4). Multivariate logistic regression analysis revealed the combined and individual associations between physicians’ demographic and professional characteristics with their odds to reverse the code status of their SMA patients in light of impending compromise. Junior physicians were significantly more likely to reverse the DNR status of these patients on average, accounting for the other physicians’ characteristics (OR=1.6; 95% CI=1.174-2.439; p=0.005). Physicians of other specialties like pediatric intensivists differed significantly in their odds of reversing the code status of their patients (OR=0.690; 95% CI=0.487-979; p=0.038) (Table 5) (Figure 1&2).
Physicians’ reported aspects and practices of SMA patients care (N=169).
Multivariate logistic regression analysis of the combined and individual associations between physician demographic and professional characteristics with their odds to reverse the code status of SMA patients in light of impending compromise (N=168).
Correlation of physicians’ current position with reported probability of reversal of the DNR status. Fellow: pediatric and adult neurology fellow in training program.
Correlation of physicians’ main field of practice with reported probability of reversal of the DNR status.
Discussion
Historicaly, SMA patients have poor outcome. With recent advances in the management of these patients especially with the appearance of new therapies, these patients have an extended lifespan.13 Many questions arise about the best way to the management of the children with SMA and the way to provide the patient and their families with the best quality of life and resources. Answering these questions at a single institution and based on personal experience is difficult and scientifically not appropriate. Spinal muscular atrophy is a rare neurodegenerative disorder, and evidence-based guidelines for management of this condition is lacking. In 2007, the first international consensus guidelines were published.7 These consensus guidelines were revised and published during the time of this project.14,15 The present study showed that 50.3% of the participants were aware of the consensus guidelines (Likert score=6.6). This result was in agreement with a previous study.16 As expected, the field of specialty of experienced physicians was a significant predictor of their awareness with the consensus; pediatric neurologists were significantly more inclined to be aware of the consensus when compared to pediatric intensivists.
Nusinersen is approved for the treatment of SMA in the United States of America, the European Union, Brazil, Japan, Switzerland, and Canada. It started globally in 2016 in response to the urgent need for treatment for the most severely affected individuals living with SMA.17 The current study showed that half of the physicians were uncertain about the use and benefit of this medication. Another study describing the current practice in the care of SMA in Canada reported that half of the participants believed that a disease-modifying therapy was likely to be available within 10 years.18
There was wide variability in clinical practice suggested by this study. Over half of the physicians (53.7%) advised that DNR and “no intubation” were implemented in their hospital, followed by full supportive care (32.9%), tracheostomy and home ventilation (30.5%), and non-invasive ventilation therapy (NIV) (26.8%). There was a wide variability between physicians in clinical practice suggested by this study, demonstrated in the attitudes of the physicians towards modality of ventilation in SMA patients and code status. This diversity of therapeutic practice may be partially due to the lack of awareness of the international consensus guidelines especially among intensivists caring for these patients.19 This study showed that almost two-thirds of the physicians (59.8%) were aware of gene-specific therapy as a promising treatment for SMA, while the minority were aware of possible roll of stem cell transplantation. This variation may be attributed to cultural differences and variable administrative and legal issues. A new study using single-dose gene therapy in infants with type I SMA conducted by Mendell R et al,20 about the use of gene therapy in 15 infants with type I SMA. Although the primary outcome measure was the safety of the therapy, all 15 infants were alive at the age of 20 months; the outcome of the study was encouraging.20 In our study, when physicians were asked to reverse the code status of their SMA patients in light of impending compromise, junior physicians were significantly more likely to reverse the DNR status of these patients, while physicians with various expertise differed significantly. When the physicians were asked about their belief in the ability of Nusinersen to improve SMA outcomes, the majority were uncertain about reversing the code status of applying DNR to SMA patients, and they were unsure of the diversity of therapeutic attitudes. Due to lack of experience of junior physicians and the awareness of expert physicians, this new modality of treatment is still under trial. Confirmation of its long-term efficacy and the legal issues involved will require time. In the other hand, early use of this medication is very crucial and has favorable outcome. Moreover, the high cost of this new therapy must be considered, especially in low-income countries.
Limitations
Relatively small number size and different specialties and level of experience.
In conclusion, our study identified wide differences in physicians’ practice toward management of children with SMA. Our data demonstrate a need for increased awareness of consensus guidelines for standard of care for patients with SMA. Furthermore, physicians’ attitudes towards and knowledge of novel modalities of treatment were found to be a challenge in the management of SMA.
Acknowledgment
The authors would like to thank Mr. Mohamad A. AlKhateeb for statistical analysis.
Footnotes
Disclosure. This study was funded by the college of Medicine Research Center, Deanship of scientific research, King Saud university, Riyadh, Kingdom of Saudi Arabia.
- Received August 16, 2018.
- Accepted October 3, 2019.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.