Abstract
Objectives: To compare the efficacy and safety of corpus callosotomy versus vagus nerve stimulation (VNS) as long-term adjunctive therapies in children with Lennox–Gastaut syndrome.
Methods: This retrospective study was conducted in King Fahad Medical City between 2010 and 2019. The authors identified and followed 9 patients with Lennox–Gastaut syndrome (LGS) who underwent corpus callosotomy or VNS implantation for at least 12 months; seizure frequency and major complications were monitored. Five patients with a mean age of 10.8±1.3 years had corpus callosotomy, and 4 patients with a mean age of 13.8±3.9 years were implanted with VNS stimulators.
Results: Reduction in seizure frequency was achieved in all 5 patients who underwent corpus callosotomy, with greater than 75% seizure reduction in more than 50% in one, and greater than 25% in 2 respectively. However, in those implanted with VNS, 2 (50%) patients achieved a reduction in seizure frequency of greater than 75% and 2 (50%) greater than 25%, respectively. No significant difference was observed between the 2 treatment groups. One patient who underwent corpus callosotomy suffered cerebrospinal fluid leakage, and swallowing difficulties in one patient who underwent VNS.
Conclusion: Both corpus callosotomy and VNS are safe and effective as adjunctive treatments for LGS patients.
Lennox–Gastaut syndrome (LGS) is a severe form of childhood-onset epilepsy that was first described by Dr. Henri Gastaut in 1966.1 In 1950, Dr. William G. Lennox described the electroencephalogram features of this condition.2 It is characterized by a triad of several types of seizures, cognitive impairment, and a distinctive awake interictal EEG pattern of diffuse slow basic rhythm interrupted by spike–wave complexes (1.5–2.5 Hz) while non-REM sleep characterized by discharges that are more generalized and more frequent, comprising polyspikes and slow waves; this condition is considered to be an epileptic encephalopathy.3-6 Lennox–Gastaut syndrome accounts for approximately 2-5% of all childhood epilepsies.7-8
Particularly in children, the incidence is estimated at 2 per 100,000,9 and it is more common in males.10 The LGS is one of the most commonly encountered drug-resistant epileptic encephalopathies, with mortality rates ranging between 3% and 7%. Moreover, the history of infantile spasms, or West syndrome, has been associated with an unfavorable prognosis.11-12 There are many treatment options for LGS patients, such as antiepileptic medications, ketogenic diets, VNS therapy, corpus callosotomy, and resective epilepsy surgery.11 Systematic review and meta-analysis showed that Cannabidiol (CBD) has an antiseizure mechanism in adults and children with drug-resistant epilepsy. Furthermore, it shows a significant reduction. in drop, non‑drop, and All Seizures frequency. However, it has not yet been approved by the Saudi Food and Drug Authority.12
Due to the difficulty of identifying and localizing single epileptogenic lesions and the presence of multifocal characteristics, most LGS patients are ineligible for resective surgery.11 Palliative surgical procedure for refractory epilepsy, such as corpus callosotomy, was introduced in the 1940s by Van Wagenen and Herren.13 Since then, it has been widely used and indicated for different types of intractable generalized seizures.14 On the other hand, VNS therapy was first performed in 1988.15 It was approved by the US Food and Drug Administration as an adjunctive therapy for drug-resistant epilepsy in those who are not surgical candidates.16
Both procedures can be used to reduce seizure frequency and severity in LGS patients.17,18 The corpus callosum is a bundled white matter fiber that connects both hemispheres to allow slow brain waves to travel back and forth between both cerebral hemispheres during slow-wave sleep (SWS). Avvenuti et al suggest that having a well-connected corpus callosum is essential for the cross-hemispheric dispersion of slow waves during sleep slow waves, non-rapid eye movement (NREM) sleep. According to the authors, this is the first time that sleep researchers have identified that non-REM sleep “slow waves” travel between brain hemispheres via so-called “anatomical highways” in the corpus callosum.19
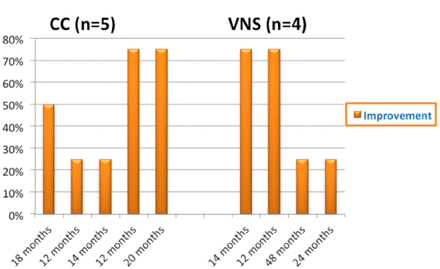
- Improvement (%) during follow-up (months) for the 2 groups. Improvement (%) during follow-up (months) for the 2 groups. VNS - Vagus nerve stimulation, CC - Corpus callosotomy
- Demographic analysis and clinical characteristics of the study subjects.
- Comparison of seizure reduction rates (all seizure types) and documented complications of patients treated with corpus callosotomy and vagal nerve stimulation.
Our study evaluates the efficacy and safety of corpus callosotomy versus VNS as long-term adjunctive therapies in children with LGS in King Fahad Medical City. To our knowledge and as revealed by a search of the relevant literature, this study is a unique of its novelty in Saudi Arabia and the Middle East.
Methods
This retrospective study was approved by the Institutional Review Board (IRB log number 19-012) of King Fahad Medical City. In total, we studied 9 children with LGS, whose mean ages were between 10.8±1.3 and 13.7±3.9 years. They were divided into two groups and treated with either corpus callosotomy or VNS at the Epilepsy Monitoring Unit at King Fahad Medical City. All patients had pharmacoresistant epilepsy and their main number of antiepileptic drugs (AEDs) in either groups, whether before or after surgery was almost same and the difference was not significant. An epileptic focus could not be resected in any of the patients. All the subjects underwent either corpus callosotomy or VNS between 2010 and 2019, and all received follow-up for at least 12 months. The medical records and seizure diaries of all the subjects were retrospectively reviewed for their demographic characteristics (age, gender, age of onset, seizure frequency, seizure duration before surgery, and follow-up duration), clinical characteristics (primary seizure type, brain magnetic resonance imaging [MRI] lesions, number AEDs before and after surgery, compliance with the regimen, and any change in AEDs). Complications arising from the procedures mentioned above were reviewed.
Corpus callosotomy procedure
All patients in this group underwent complete corpus callosotomy comprising anterior and posterior corpus callosum surgical resection.
VNS parameters
For the patients in this group, a VNS stimulator was implanted in the left anterior chest wall under the skin. The tool wire was wrapped inside the neck around the left vagus nerve. The VNS device was activated 1–2 weeks after implantation of the stimulator. The stimulation was started with the following parameters: an output current of 0.25 mA, a magnet current of 0.50 mA, a pulse width of 500 µs, a signal on time of 30 s, and a signal off time of 5 min. The output current and magnet current intensities were increased by 0.25 mA, as tolerated, until 2.0 mA when seizure control was insufficient, the signal off time was adjusted from 5 to 1.8 min.
Statistical analysis
As the sample size was small, the proportional differences between the 2 groups were measured by a Fisher’s exact test. The mean difference between the 2 groups was measured by a student’s t-test. The inferences were drawn at the 95% confidence interval, and a p-value of less than 0.05 was considered statistically significant.
Results
This retrospective study was approved by the Institutional Review Board (IRB log number 19-012) of King Fahad Medical City. In total, we studied 9 children with LGS, whose mean ages were between 10.8±1.3 and 13.7±3.9 years. They were divided into 2 groups and treated with either corpus callosotomy or VNS at the Epilepsy Monitoring Unit at King Fahad Medical City. All patients had pharmacoresistant epilepsy and their main number of antiepileptic drugs (AEDs) in either groups, whether before or after surgery was almost same and the difference was not significant. An epileptic focus could not be resected in any of the patients. All the subjects underwent either corpus callosotomy or VNS between 2010 and 2019, and all received follow-up for at least 12 months. The medical records and seizure diaries of all the subjects were retrospectively reviewed for their demographic characteristics (age, gender, age of onset, seizure frequency, seizure duration before surgery, and follow-up duration), clinical characteristics (primary seizure type, brain magnetic resonance imaging [MRI] lesions, number AEDs before and after surgery, compliance with the regimen, and any change in AEDs). Complications arising from the procedures mentioned above were reviewed.
Corpus callosotomy procedure
All patients in this group underwent complete corpus callosotomy comprising anterior and posterior corpus callosum surgical resection.
The VNS parameters
For the patients in this group, a VNS stimulator was implanted in the left anterior chest wall under the skin. The tool wire was wrapped inside the neck around the left vagus nerve. The VNS device was activated 1–2 weeks after implantation of the stimulator. The stimulation was started with the following parameters: an output current of 0.25 mA, a magnet current of 0.50 mA, a pulse width of 500 µs, a signal on time of 30 s, and a signal off time of 5 min. The output current and magnet current intensities were increased by 0.25 mA, as tolerated, until 2.0 mA when seizure control was insufficient, the signal off time was adjusted from 5 to 1.8 min.
Statistical analysis
As the sample size was small, the proportional differences between the 2 groups were measured by a Fisher’s exact test. The mean difference between the 2 groups was measured by a student’s t-test. The inferences were drawn at the 95% confidence interval, and a p-value of less than 0.05 was considered statistically significant.
Discussion
Both surgical procedures are effective treatment modalities in children and adolescents with LGS. However, corpus callosotomy could be an effective primary choice if atonic (drop attack) seizures are the main seizure type. On the other hand, VNS is preferred if myoclonic seizures, generalized tonic–clonic, or atypical absence seizures are the prominent seizure types. Both procedures have shown comparable efficacy. Indeed, the lower morbidity and complication rates reported with VNS implantation make it the first-line treatment.20
Maehara et al21 reported seizure cessation or a 90% greater reduction in drop attacks in 44 of 52 patients (85%) who underwent total corpus callosotomy and were followed up for at least 40 months. Both surgical treatments are safe for atonic seizures, but corpus callosotomy shows more promise in the treatment of this highly morbid seizure type.22 The VNS therapy has been shown to reduce seizure rates by 50% or more in about 25% of patients and an average of approximately 20–30% in the remaining patients.23-24 A large retrospective study involving 50 LGS patients who received VNS therapy from 6 centers showed median reductions in total seizures of 42% at one month, 58.2% at 3 months, and 58% at 6 months; no significant complications were documented.25
You et al18 reported 24 patients with LGS, 14 of whom underwent corpus callosotomy, and 10 of whom received VNS implantation. The primary seizure type in all the patients was drop attack (tonic or atonic). According to the results, 64.3% of the corpus callosotomy patients exhibited a reduction in seizure frequency of more than 50%, while 70% of patients in the VNS group showed the same reduction with few tolerable transient complications. Recently, the effectiveness and responsiveness of a combined intervention, namely, VNS following corpus callosotomy, in patients with LGS was studied by Katagiri et al26 where he reported that 60% of the patients had a successful seizure reduction rate of 50% or more for residual seizures regardless of seizure type (except for drop attacks). Moreover, 20% of the patients were seizure-free at 12 months post-VNS. Indeed, excellent seizure outcomes for patients suffering from drop attacks were achieved after corpus callosotomy in 77.8% of patients with drop attacks. Our data revealed that corpus callosotomy and VNS were similarly effective in reducing seizures in refractory LGS patients if patients were appropriately selected as per the modalities of the administered treatments. The complication rate in our study was similar in both groups, although the corpus callosotomy was associated with more severe complications than those in the VNS group.
Our study was a nonrandomized retrospective case series involving 9 patients with Lennox–Gastaut syndrome. In our analysis, all the patients showed seizure reductions to variable extents. The primary type of seizure was head-drop in most patients in both groups. Despite the interesting results, this research has some limitations and due to the rarity of this condition, the number of patients was small, and therefore the statistical tests were less powerful. Other limitations include differences in duration of seizures before surgery and length of follow-up after treatment.
Conclusion
Our data revealed no significant differences in the efficacy and safety of corpus callosotomy versus VNS as a treatment for children with LGS. However, the safety profile was more favorable in the VNS group. More extensive research studies with a larger number of patients are needed to evaluate the most beneficial therapeutic procedure for patients with LGS.
Acknowledgement
The authors would like to thank Dr. Gehadd Al hajaj, epilepsy fellow for his support during data collection and many thanks for Mr. Tariq Wani, for facilitating statistical analysis. The authors would like to thank also Scribendi company for English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received October 24, 2021.
- Accepted January 11, 2022.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.