Abstract
Guillain–Barré syndrome (GBS) has several clinical variants. The sensory presentations of GBS are atypical but well-recognized. We report a patient who presented with predominantly sensory symptoms associated with reversible conduction failure (RCF). RCF is a well-defined neurophysiological abnormality noted mainly in axonal GBS and may be misinterpreted as evidence of demyelination. A 25-year-old woman presented 2 weeks after a coronavirus 2019 infection with acute sensory symptoms, distal allodynia, mild weakness, and mild hyporeflexia in her upper limbs. A nerve conduction study (NCS) showed delayed motor distal latencies, and lumbar puncture confirmed cytoalbuminologic dissociation. After excluding other etiologies, she was diagnosed with GBS, treated with an IV immunoglobulin course, and showed remarkable recovery. Results of a repeat NCS were consistent with RCF and confirmed the presence of axonal GBS. Increased awareness of sensory GBS and RCF is expected to improve the diagnosis and management of atypical GBS presentations.
Guillain–Barre syndrome (GBS) has many variants, and most of these variants have been reported post-coronavirus disease 2019 (COVID-19).1 Pure or predominantly sensory neuropathy is a well-recognized, but a controversial variant of GBS.2,3 We report a patient who developed predominantly sensory GBS, two weeks after testing positive for respiratory syndrome coronavirus 2 (SARS-CoV-2) and showed neurophysiologically reversible conduction failure (RCF). Reversible conduction failure is a rapidly reversible conduction block without remyelination-related changes and is consistent usually with axonal GBS.4
Case Report
Patient information
A 25-year-old woman presented with a 1-week history of acute tingling and pain sensations in the face, tongue, hands, and feet. The pain was persistent, described as burning pain involving mainly the hands and feet, and exacerbated by touch and walking. She also experienced mild walking difficulties and weakness. Two weeks earlier, she was diagnosed with COVID-19 after developing mild symptoms of fever, headache, and general malaise that lasted for a few days, and recovered well. Apart from the positive polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), all her basic blood tests were normal. She was not vaccinated against COVID-19. Otherwise, past and family histories were unremarkable.
Clinical findings
Physical examination revealed mild truncal ataxia and grade 4+/5 weakness on the Medical Research Council scale, involving finger and wrist flexion and extension and ankle dorsiflexion, and plantar flexion. Proximally, however, power was normal and muscle stretch reflexes were only mildly diminished in the upper limbs and normal in the lower limbs. Sensory examination was remarkable for tongue and perioral paresthesia, severe tactile allodynia afflicting the hands more than the feet, with mild diminution of pinprick and vibration sensations involving both hands and feet. (Table 1)
- Timeline of the patient’s clinical condition.
Diagnostic assessment
A nerve conduction study (NCS) showed delayed distal latencies (DLs), diminished compound motor action potentials (CMAPs), and sensory action potentials (SNAPs) in the upper limbs. These changes were consistent with distal conduction block. Nerve conduction velocities and F-wave latencies were within normal in all motor nerves. Lumbar puncture revealed a high cerebrospinal fluid protein of 115 mg/dL (15–45 mg/dL) and a normal white cell count of 3 cells/µL (<5 cells/µL). Other tests included normal sedimentation rate; C-reactive protein; vitamin B1, B6, and B12 levels; Brucella IgG-IgM antibodies; negative antinuclear antibodies; and normal protein electrophoresis. She was diagnosed with probable demyelinating GBS, occurring post-COVID-19 infection.
Therapeutic intervention
She was started on gabapentin 300 mg thrice a day for pain relief and was administered a course of intravenous immunoglobulin 20 g daily for 5 days.
Follow-up and outcomes
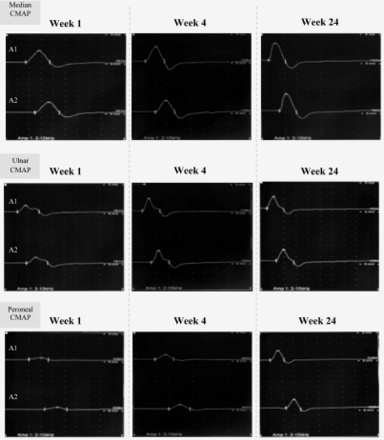
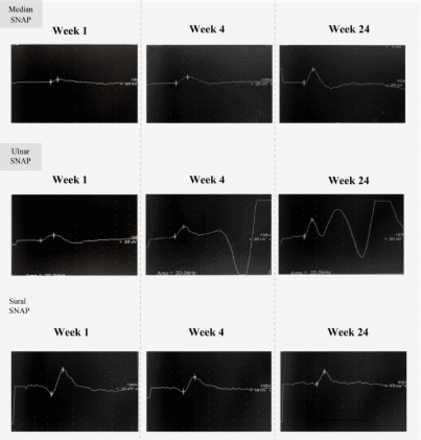
Two weeks later, she reported a gradual improvement in gait. Tongue and perioral paresthesia had already disappeared, and distal limb pain had significantly diminished. Further improvement was reported 1 month later, as she returned to near-normal daily activity. Repeated NCS on week 4 and later on week 24, showed remarkable and rapid recovery of DLs and CMAPs amplitude which improved in week 4 by more than 50% (Figure 1). Similar improvement was noted in sensory onset latencies (OLs) and SNAPs amplitude but involved the upper limbs only (Figure 2). Median nerve SNAP amplitude recovered by more than 100% within 4 weeks. This recovery was not associated with temporal dispersion which was consistent with an underlying fully reversible process of RCF indicating probable axonal GBS. On week 24 all NCS parameters normalized completely.
- Sequential CMAPs studies. Serial NCS on weeks 1, 4, and 24 showed gradual recovery of motor DLs; Median nerve: 8.6, 5.7, and 3.4 ms. Ulnar nerve: 5.5, 4.2, and 2.9 ms. Common Peroneal nerve: 10, 9.2, and 5 ms. Also show gradual recovery of CMAP amplitude at weeks 1, 4, and 24; Median nerve: 9.9, 12.7, and 14.8 mv, ulnar nerve: 5.7, 10.4, and 12 mv, common peroneal nerve: 1.6, 4 and 8.3 mv. CMAP - compound muscle action potential. DL - distal latency, ms - milli-second, mv - milli-volt
- Sequential sensory nerve action potentials studies. Serial NCS on weeks 1,4 and 24 showing mild changes in sensory OLs; Median nerve: 3, 2.4, and 2 ms. Ulnar nerve: 2.2, 2.4, and 2.1 ms. Sural nerve: 3, 3, and 3 ms. But SNAPs amplitude recovery at weeks 1, 4, and 24 was significant in upper limbs; Median nerve: 5, 15, and 36 µv. Ulnar nerve: 13,15 and 44 µv. Sural nerve: 29, 18, and 17 µv. SNAP - sensory nerve action potential. OL - onset latency, ms - milli-second, µv - micro-volt
Discussion
Sensory GBS has long been accepted as a well-recognized clinical presentation of GBS; however, its classification and definition remain controversial.2,3 Our patient conformed partially with the main criteria for GBS; a mono-phasic course, diminished muscle stretch reflexes in upper limbs, cytoalbuminologic dissociation, a consistent NCS, and the absence of an alternative diagnosis.3 Similar to other cases reported in the literature, the prominent sensory symptoms of burning pain, tingling, numbness, and allodynia together with the mild distal weakness are considered, according to these criteria atypical and cast doubt on the diagnosis. This state of uncertainty regarding sensory GBS was discussed in detail by many authorities starting with Asbury, who went on to describe a diagnostic criterion for what he labeled as sensory loss and areflexia variant.5 Uncini and Yuki proposed a classification of sensory GBS into three subtypes: small-fiber axonal neuropathy, large-fiber axonal neuropathy, and sensory demyelinating polyneuropathy.2 The first NCS in our patient was initially consistent with a demyelinating process, and there are few reports in the literature describing sensory GBS with distal demyelination.6,7 Clinically, similar to our patient, allodynia was the main presenting symptom in the patient reported by Simproni et al6 in association with facial, tongue, and hand numbness.6 In our patient, improvement in facial and tongue numbness was the first sign of recovery. Osterlund-Tauriala and Partanen reported 3 patients who also presented with predominant distal sensory symptoms, which also included the trigeminal territory.7 Weakness was mild in 2 of their patients and absent in the third patient. This clinical picture was associated with the characteristic neurophysiological abnormalities of delayed distal latencies (DLs) in the presence of normal conduction velocities, consistent with distal demyelination, which was further confirmed by sequential studies. With this characteristic neurophysiological picture, they suggested labeling this type of GBS presentation as a distal variant of AIDP.7
In our patient, the acute predominantly sensory clinical picture was associated with remarkable distal conduction slowing, manifested by prolonged DLs and diminished CMAPs amplitude. However, sequential NCS proved that these changes are not due to distal demyelination, but rather to RCF. Reversible conduction failure is a well-recognized neurophysiological phenomenon commonly associated with axonal GBS and is described as a rapid recovery of conduction block or slowing in the absence of changes that accompany demyelination-remyelination process.8 As it was noted in our patient, NCS abnormalities recovered rapidly and fully on repeated studies without the appearance of temporal dispersion, consistent with RCF. Reversible conduction failure may be related to immune-mediated inflammatory changes occurring at the nodes of Ranvier or para-nodal regions in association with anti-ganglioside antibodies.4,8 There is a growing interest in the role of CRF in GBS classification because it may be misinterpreted as evidence of demyelination. To avoid missing CRF, Uncini and Kuwabara recommended performing a second study if the initial NCS results do not show clear demyelinating features, low-amplitude distal CMAPs, or conduction blocks without temporal dispersion.8
Post COVID-19 GBS has been reported worldwide, including Saudi Arabia.1,9 In a systemic review by Abu-Rumeileh et al, the main variants of GBS were reported in association with COVID-19 infection, including acute inflammatory demyelinating polyneuropathy, acute motor axonal neuropathy, and acute motor sensory axonal neuropathy. Furthermore, most rare variants were also reported including Miller-Fisher Syndrome, pure motor, bilateral facial palsy with paresthesia, and paraparetic form.1 Our patient adds to this list of rare GBS variants occurring post-COVID-19 infection and serves as another example of the exceptional autoreactive effects of the SARS-CoV-2 virus on the immune system.10
Conclusion
We report the first case of a rare predominantly sensory variant of GBS, occurring post-COVID-19 infection. It was associated with RCF, an easily misinterpreted neurophysiological finding as evidence of demyelination. Increased awareness of CRF is expected to improve GBS classification.
Acknowledgement
The authors would like to thank Editage.com for their assistance in English language editing.
- Received May 24, 2022.
- Accepted October 26, 2022.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.