Abstract
Carpal tunnel syndrome (CTS) is the most common median nerve neuropathy, accounting for 90% of all neuropathies. Carpal tunnel syndrome presents in 3.8% of the general population, with a higher prevalence among women. There are several risk factors associated with CTS, including both medical and non medical factors. The pathophysiologic mechanisms involved in the median nerve compression and traction are thought to be complex, and as yet are not fully understood. The present review aimed to provide an overview of the pathophysiology of median nerve neuropathy in the carpal tunnel, and subsequent development of CTS.
The carpal tunnel is an osteofibrous outlet, which lies between the flexor retinaculum (FR) and the carpal bones. The roof of this tunnel is the fibrous transverse carpal ligament, which is the intermediate part of the FR. Nine flexor tendons, and their sheath, and the median nerve pass through the tunnel where the nerve enters the tunnel in the midline or slightly radial to it.1 The median nerve gives sensory branches to supply the 3 radial digits and the radial half fourth digit. It also gives the palmar sensory cutaneous branches, which supply the cutaneous skin of the palm,2 and the thenar (recurrent) motor branch. Carpal tunnel syndrome (CTS) is a neuropathy caused by compression and traction of the median nerve at the level of the carpal tunnel, delimitated by the carpal bones and by the transverse carpal ligament (FR).3 Physiological evidence indicates increased pressure within the carpal tunnel (not only CTS is due to combined compression and traction of the median nerve), and therefore decreased function of the median nerve at that level.4 Carpal tunnel syndrome was first described by Paget in 1854.5 Since that time, CTS was commonly noted in the medical literature, and remains one of the most common disabling conditions presented to orthopedic and rheumatology clinics.3 Carpal tunnel syndrome is the most well-known and frequent form of median nerve neuropathy,6,7 and accounts for 90% of all neuropathies.8 It affects 4-5% of the population especially between the ages of 40-60 years. The prevalence rate is higher among females (9.2%) than among males (6%) between the ages of 45-60 years.8,9 Although most cases of CTS are idiopathic, CTS is mainly due to a fibrous hypertrophy of the synovial flexor sheath, and to repetitive movements of the wrist. Certain risk factors have been reported to be associated with CTS,10 which include medical and occupational factors. The medical factors could be divided into extrinsic, intrinsic, and neuropathic factors. Furthermore, the etiologies of CTS could be classified according to Chammas6 as idiopathic and secondary CTS. Most cases of CTS are considered to have no specific cause and are defined as idiopathic cases. Several risk factors such as gender, age, genetic, and anthropometric factors (size of the carpal tunnel) have been implicated in the risk of idiopathic CTS.6 Occupational risk factors of repetitive tasks, force, posture, and vibration have been cited as a significant risk factor of CTS development,11 and some industries such as fish processing have reported the prevalence of CTS in their workers to be as high as 73%.12 A variety of extrinsic and intrinsic factors that can cause abnormalities in the contents or in the containers of CTS can increase the risk of CTS and result in secondary CTS. Of these factors; pregnancy, menopause, obesity, renal failure, hypothyroidism, the use of oral contraceptives, and congestive heart failure can increase the risk of CTS through increasing the volume of the synovial sheath within the tunnel.13 Also, fractures of the distal radius, directly or through posttraumatic arthritis, and the heredity small size carpal tunnel can alter the contour of the tunnel.13,14 Intrinsic factors within the nerve that increase the occupied volume inside the tunnel include tumors and tumor-like lesions.15,16 Neuropathic factors, such as diabetes, alcoholism, vitamin toxicity or deficiency, and exposure to toxins, can play a role in eliciting CTS symptoms. Diabetic patients have a higher tendency to develop CTS with a prevalence rate of 14% without, and 30% with diabetic neuropathy.17 The pathophysiologic mechanisms involved in median nerve compression and traction; however, are thought to be complex and as yet are not fully understood. The present review aims to provide an overview of the different pathophysiologic mechanisms involved in the median nerve neuropathy and CTS development.
Pathophysiology of CTS
The entrapment neuropathy combines phenomena of compression and traction. Nerve compression and traction may cause disorders of the intraneural microcirculation, lesions in the myelin sheath and the axon, as well as alterations in the supporting connective tissue. The entrapment of a peripheral nerve occurs as a result of its passage through an anatomical compartment that has become too tight, resulting in altered function within the nerve and dysfunction/damage of the nerve from the site of compression and beyond.18 Median nerve entrapment in the carpal tunnel at the wrist is the most common example of this. The available literature has indicated a combination of several pathophysiologic mechanisms in CTS. These mechanisms are interacting and include the increased pressure in the tunnel, median nerve microcirculation injury, median nerve connective tissue compression, and synovial tissue hypertrophy.
In addition to these mechanisms, the results of post-operative outcome of carpal tunnel release reported by Ozkul et al,19 indicated that a less favorable outcome was found among diabetic when compared with non diabetic patients. These results suggested that CTS in diabetic patients may also stem from internal factors deleterious to the nerve including hyperglycemia and deficiency of neurotrophic factors such as nerve growth factor.19
Increased carpal tunnel pressure
Anatomically, there are 2 sites of median nerve compression: 1) At the proximal edge of the carpal tunnel, caused by wrist flexion and due to the change in thickness and rigidity between the antebrachial fascia and the proximal portion of the FR; and 2) At the narrowest portion at the hook of hamate. Normal pressure in the carpal tunnel has been recorded to range from 2 to 10 mm Hg.20 Dramatic changes of the fluid pressure in the carpal tunnel have been reported with wrist movement, with wrist extension increasing the pressure by 10-fold, and flexion increasing it by 8-fold.20 Bauman et al21 utilized a wick catheter to demonstrate that the tunnel pressure was higher in idiopathic CTS patients than in normal subjects. In neutral wrist posture, the average pressure that is registered in the patient’s carpal canal is 32 mm Hg. When the wrist is flexed, the pressure reaches a value of 94 mm Hg, but is 110 mm Hg when the wrist is extended. Pathological changes occurring in the ligaments surrounding nerves including alterations in the amount and flexibility of connective tissue are thought to be the basis for increased pressure. Experimental studies have suggested a dose-response relationship between median nerve dysfunction, and the duration, and amount of carpal tunnel compression.22 Increased carpal tunnel pressure is thought to cause ischemic compression of the median nerve, and a number of experimental studies support the theory of ischemia due to externally applied compression and due to increased pressure in the carpal tunnel.23 Seiler et al24 reported, by using laser Doppler flowmetry, the restoration of normal pulsatile blood flow within the median nerve within one minute of transverse carpal ligament release. In idiopathic CTS, the nocturnal increase in the tunnel pressure could result from several factors that include: redistribution of the upper limb fluids in supine position; lack of muscle pump mechanism that contributes to the drainage of interstitial fluid in the carpal tunnel; tendency to place the wrist in flexion thereby increasing intracanalicular pressure; increased blood pressure in the second half of the night; and fall of cortisol level.6
Median nerve microcirculation injury
Ischemic vascular injury and the breakdown in the blood-nerve barrier have also been identified as an essential component in CTS. The blood-nerve-barrier is formed by the inner cells of the perineurium and the endothelial cells of endoneurial capillaries that accompany the median nerve through the carpal tunnel. These endoneurial microvessels are formed from nutrient branches that arise from the radial and ulnar arteries, proximal to the flexor retinaculum.25 An increase in pressure within the tunnel can cause a breakdown of vasculature within this barrier, causing an accumulation of proteins and inflammatory cells.25 This may induce a miniature closed compartment syndrome by increasing the permeability, contributing to increased endoneurial fluid pressure and development of an intra-fascicular edema.26 Patients with vascular problems or prolonged exposure to static loading are particularly prone to a breakdown in the blood-nerve-barrier.27
MacKinnon and coworkers have also described common progressive neurovascular changes occurring in a series of experimental studies looking at histological findings at common sites of entrapment.28-30 These included early perineurial and endoneurial microvessel thickening with basement membrane reduplication, Renaut’s body formation, perineurial and epineurial fibrosis, and patchy fibre loss associated with thinning of myelin, attributed to fibre demyelination and degeneration.
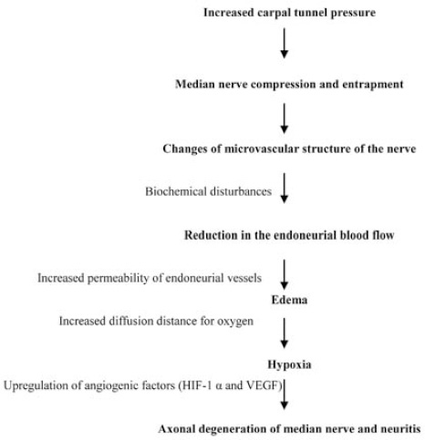
One final etiological reason for the increased susceptibility of median nerve compression, particularly in diabetic patients, is changes in the microvascular structure of the nerve, exacerbated by biochemical disturbances, which could lead to a reduction in the endoneurial blood flow and oxygen tension.31 Focal compression led to localized intraneural circulatory changes and increased permeability of endoneurial vessels as well as causing edema of the endoneurial space. The development of edema may lead to an increase in the diffusion distance for oxygen from the capillaries, which could lead to hypoxia.31 The resultant hypoxia could drive the upregulation of several angiogenic factors including hypoxia-inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF). Increased VEGF levels have been demonstrated in neurones and Schwann cells of experimental diabetic animal models.32
Furthermore, endoneurial vessels in diabetic patients undergo characteristic microangiopathic changes such as hyaline thickening and increased deposition of a Per-Arnt-Sim (PAS)-positive substance within their walls. They also display endothelial hypertrophy, hyperplasia, basement membrane thickening, and pericyte loss. The thickened vessel wall, alongside the increased endoneurial vascular permeability and edema, would also increase the diffusion distance for oxygen to reach the nerve fibres and thereby induce more hypoxia.32 A schematic presentation for vascular mechanism of CTS and median nerve injury is presented in Figure 1.
A schematic presentation for vascular mechanism of carpal tunnel syndrome and median nerve injury. HIF-1α - hypoxia-inducible factor 1α, VEGF - vascular endothelial grown factor
Median nerve connective tissue alterations
Nerve fibres have layers of connective tissue surrounding it. These layers are the mesoneurium (not included in the peripheral nerve sheath), epineurium, perineurium, and endoneurium; which is the most intimate layer. The extensibility of these layers is critical to nerve gliding (nerve gliding properties are due to the integrity of epineurium only), which is necessary to accommodate joint motion; otherwise, nerves are stretched and become injured.33 It is estimated that in normal subjects, the median nerve at the wrist can move up to 9.6 mm between full flexion and extension of this joint but in the presence of stiff surrounding connective tissue, this is limited and exposes the nerve to shearing forces that could lead to injury.19
The longitudinal movement of the median nerve in the carpal tunnel was found to be 9.6 mm during flexion, and 0.7-1.4 cm in wrist extension. It can vary from 2.5 to 19.6 mm depending on the position of the shoulder, elbow, wrist, and fingers. The median nerve tension varies from 8% depending on the position of the shoulder, and 19% depending on the position of the fingers. In addition to the longitudinal movement, a transverse movement of the median nerve occurs with wrist position or during finger flexion against resistance. In compression and epineural adhesions, mobility is hindered, creating lesions due to repeated traction on the nerve during wrist movements.
The complexity of the mechanisms underlying nerve compression and traction was described by Lundborg and Dahlin,34 who emphasized how a chain of events may set up a vicious cycle leading to nerve injury. A chronic increase in pressure in the nerve trunk produces a pressure gradient, which redistributes components of compressed tissue towards the side not compressed, with the subsequent stretching of the epineurial and vascular structures. The rapid development of edema, predominantly in the epineurium, leading to nerve swelling, would further restrict the movement of the nerve within the already narrow anatomical compartment. This scenario limits nerve gliding during movements of the extremities, further subjecting the nerve to more irritation, increased pressure on the nerve trunk and edema, initiating a vicious cycle.
Human studies of entrapment neuropathies during surgery or autopsies have found the nerves to be narrowed at the site of compression, whilst appearing enlarged at the proximal and distal segments.35 Demyelination of the nerve develops in the compression site, and can then spread to the entire internodal segment, leaving the axons intact. A block of nervous transmission ensues (neuropraxia). If the compression persists, bloodflow to the endoneural capillary system may be interrupted, leading to alterations in the blood-nerve barrier, and development of endoneural edema. This starts a vicious cycle consisting of venous congestion, ischemia, and local metabolic alterations.27 Axonal degeneration, macrophage attraction, and activation, release of inflammatory cytokines, nitric oxide, and development of chemical neuritis are all consequences of this viscous cycle if it continues for a substantial amount of time.36 The next stage includes axonal interruption, and distal Wallerian degeneration. Following surgical decompression, sensory recovery is delayed and depends on axonal regeneration capabilities (one mm/day).
Synovial tissue hypertrophy
Hypertrophy of the synovial tissue of the flexor tendons can also increase the pressure in the carpal tunnel and result in the development of CTS.37 Several histological and biochemical studies38,39 have reported tenosynovitis as a closely related risk factor to the development of idiopathic CTS. This has been confirmed by the presence of increased expression of prostaglandin E2 and VEGF in synovial biopsy tissue from patients with symptomatic CTS.40 In response to this injury, there is an increase in fibroblast density, collagen fibre size, vascular proliferation, and type III collagen in the synovial connective tissue.41
Constrictive scar tissue would be formed around the median nerve,42 which in turn can result in tethering of the nerve. The inflammatory thickening of the synovial tissue increases the volume of tissue which in turn increases the fluid pressure within the carpal tunnel.43 The most profound thickening of synovial tissue has been reported to be at the entrance and exit regions of the canal where the tendons slide over a fulcrum of the flexor retinaculum.43 Strain and micro-damage to the synovial tissue as well as the median nerve can occur due to the different degrees of excursion between the flexor tendons and the median nerve.39,44,45 These structural changes are aggravated by diabetes mellitus as non-enzymatic glycosylation of collagen is increased in diabetes, resulting in the alteration of packing, cross-linkage, and turnover of collagen. Increased glycosylation adversely affects collagen degradation, resulting in the accumulation of less compliant connective tissue, and ultimately fibrosis.46 An increase in lysyl oxidase activity, an enzyme involved in collagen cross-link formation that adds to the fibrosis and stiffness, has also been suggested to play a role.47 This may lead to an increase in the intra-compartmental pressure and restrict the peripheral nerve gliding movement between tissues.
Also, double crush syndrome, was described by Upton and McComas,48 who initially proposed that focal compression of an axon often occurs at more than one level. They postulated that nonsymptomatic impairment of axoplasmic flow at more than one site along a nerve might summate to cause a symptomatic neuropathy. This was suggested by clinical observation that the majority of patients had a median neuropathy associated with evidence of cervicothoracic root lesions. Other researchers have since reported series of patients supporting the frequent association of a proximal and distal nerve compression syndrome, including CTS associated with cervical radiculopathy, brachial plexus compression, and diabetic neuropathy.49 MacKinnon and Dellon50 have also expanded the description of this syndrome to include a) multiple anatomic regions along a peripheral nerve, b) multiple anatomic structures across a peripheral nerve within an anatomic region, c) superimposed on a neuropathy, and d) combinations of the above.50
In conclusion, CTS is a constellation of symptoms associated with the compression and traction of the median nerve in the carpal tunnel. The pathophysiology of CTS is complex and results from interactions of many mechanisms. However, the different pathophysiologic mechanisms presented in this review demonstrate that abnormally high carpal tunnel pressure and traction neuropathy is most likely to induce CTS. Compression and traction cause obstruction to venous outflow, edema formation, and ultimately, ischemia and nerve injury. Further studies, particularly human studies, of these interacting pathophysiologic mechanisms are necessary to shed more light not only on pathogenesis but also on the prevention and control of this disabling disease.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.