Abstract
Endovascular repair of blunt aortic injury is now a first-line approach in management. This can warrant coverage of the left subclavian artery (LSA), which could lead to posterior strokes. In this case report, we present a severe complication of endovascular repair of a traumatic aortic aneurysm. A 53-year-old man presented with blunt aortic injury, endovascular repair was carried out where the left subclavian artery was covered. The intervention had a 100% technical success. Twelve hours later, he was discovered to have quadriplegia, a CT scan showed a large left cerebellar infarction extending to the medulla oblongata and proximal spinal cord. Strokes complicate 3% of thoracic endovascular aortic repairs, 80% of those strokes occur in patients who had their LSA’s covered. Most patients however, tolerate the coverage. Although our patient had a dominant right vertebral artery, and lacked risks for these strokes, he developed an extensive stroke that left him quadriplegic.
In spite of the lack of strong prospective evidence, thoracic endovascular aortic repair (TEVAR) as an approach to manage blunt aortic injury (BAI) is rightfully making its claim in the surgical community as a first-line approach to the repair, being significantly less incident of the ominous neurological complications; namely stroke and spinal ischemia.1 However, left subclavian artery (LSA) coverage needed for fixation in 40% of TEVARs performed has paved the way for posterior circulation strokes being relatively more significant as far as complications are concerned.2,3 Although most patients can tolerate the coverage, some cannot, and the emergent situation that is BAI in many cases can only complicate matters all the more.4 Here, we present a case where this repair resulted in a drastic complication; an extensive stroke that left the patient quadriplegic. In presenting this case, we highlight the unpredictability of these complications and the lack of clear guidelines in the approach to lower such complications.
Case Report
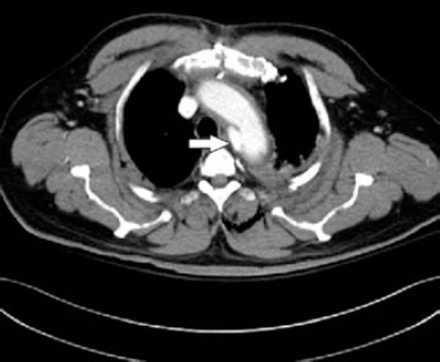
A 53-year-old man, with a history of type-2 diabetes mellitus, hypertension, and a sternotomy to remove a bullet from a gunshot injury, was involved in a motor vehicle accident. He was initially stabilized, thoroughly examined, and was cleared of cervical spinal injury at a local primary health-care center. He was then transferred to our hospital. He was hemodynamically stable. A CT-angiography scan showed a complex transection with an irregular lobulated pseudoaneurysm in the undersurface of the aorta immediately distal to the LSA, measuring 2.6 × 2.5 × 2.2 cm. The intramural aortic hematoma extended into the descending aorta, aortic arch, and all arch vessels, and showed no calcific or non-calcific atherosclerosis. The vertebral arteries arose from the proximal LSA bilaterally (Figure 1). The injury was classified as a grade III thoracic aortic injury.5 He was then scheduled for emergency thoracic endovascular repair, within 12 hours from being admitted to our facility. The operation was carried out in the endovascular suite, under general anesthesia, and continuous hemodynamic monitoring. He was placed in the supine position, with the left and right arm extended. The procedure was carried out under C-arm fluoroscopic guidance. A right-sided cutdown incision was carried out over the area of the femoral artery, while the left side was catheterized percutaneously in the standard fashion. The endovascular graft was sized and measured preoperatively (82 × 100 mm). Angiography was carried out, and we decided to cover the left subclavian as there was no sufficient landing zone for the Endograft. Because of the emergent fashion of the procedure, dominant right vertebral artery, and an intact Circle of Willis, a carotid-subclavian bypass was not considered.
Axial CT showing the lesion; complex transection with an irregular lobulated psudoaneurysm in the undersurface of the aorta immmediatly distal to the LSA, measuring 2.6 × 2.5 × 2.2 cm. LSA - left subclavian artery.
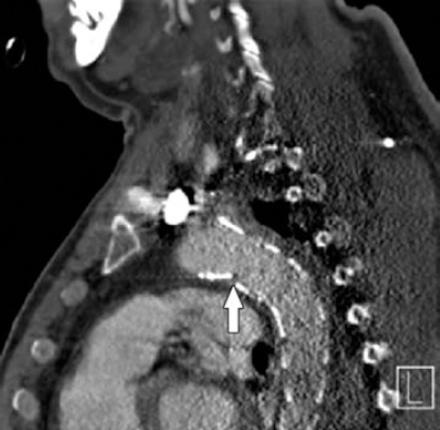
The procedure had a technical success of 100% and exclusion of the aneurysm was accomplished (Figure 2). Post-operative angiography showed minimal sluggish flow in the LSA. He received 5000 units of unfractionated heparin, and he was hemodynamically stable throughout the procedure. He was kept intubated, and a left sided chest tube was inserted to drain the hemothorax. He was then transferred to the ICU for further management where he remained hemodynamically stable and afebrile.
Left sagittal CT showing the placed stent.
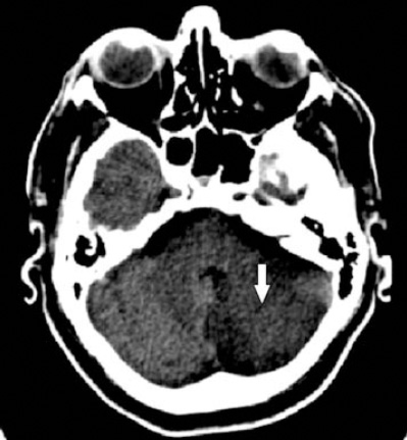
Upon attempting to wean him of the ventilator 12 hours after the procedure, it was discovered that he could not move his upper and lower limbs, with no response to painful stimuli, and unable to answer questions using eye movements. He was immediately sent for a brain CT angiogram that showed normal size right vertebral artery but attenuated left vertebral artery, meeting to form a normally-seen basilar artery with a large left cerebellar acute infarction extending into the medulla and proximal spinal cord (Figure 3). A CT neck angiogram showed 2 stents (proximal short and distal long stent) covering the orifice of the LSA and terminating in the descending aorta at the diaphragmatic hiatus, excluding the false aneurysm. Unfortunately, the excluded LSA was only partially opacified and could not be assessed further. He remains ventilator-dependent, and responds to verbal commands with his eyes.
Computed tomography of head showing large left cerebellar acute infarction.
Discussion
Aortic injury remains a common cause of death after trauma, second only to head injury; 80% of patients with BAI die before being approached for management.6 For the minority of cases that live, and whose aortic injury does not result in death by the time of presentation, repair is warranted (for grades > 2).1 Surgical repair was the convention, but TEVAR being introduced to manage acute traumatic aortic injury as recently as 1997, has proven more convenient and most importantly associated with a significant drop in morbidity and mortality.1
Strokes in TEVAR continue to plague patients with a reported incidence of 3%, and even though LSA coverage seems to be tolerated by most patients, perioperative risk of stroke is significantly increased with 80% of patients who develop strokes with TEVAR have their LSAs covered during the procedure.3,7 These strokes are mainly posterior ones (cerebellum and brainstem), which is coincident with our understanding of LSA and its distribution. Therefore, covering the LSA in a left-vertebral-dominant individual or in those with risky variants of the circle of Willis causes them to be obvious risky candidates.3 To prevent this devastating complication, LSA revascularization is carried out, but even in elective TEVARs, whether to perform it routinely or selectively remains a controversial issue.2 Plus, no procedural modifications in the literature have been widely accepted and used to avoid this complication.2 As most aortic injuries caused by blunt thoracic traumas are at the isthmus, in BAIs, covering the LSA is common. But even in these likely emergent patients, LSA coverage is well tolerated.4 Consequently, comes the majority opinion by the Society for Vascular Surgery to revascularize selectively; revascularization being individualized to each case.
In our patient, no risky anatomic variants were present; the right vertebral artery was dominant, and the Circle of Willis was complete. His blood pressure remained within the targeted range throughout the operation with no fluctuations. Other risk factors described by Gutsche et al,7 namely, prior strokes and significant atherosclerosis of the aorta on CT, were also absent in our patient. In our review of the literature, we did not encounter other cases of TEVAR where strokes resulted in quadriplegia. The one case of quadriplegia encountered was reported by Kasirajan et al,8 where a patient developed paraplegia that progressed to quadriplegia 48 hours after trauma. However, the pathology in their patient was an extensive cervical spinal ischemia.8
In conclusion, we reviewed the literature for risk factors of strokes in TEVAR and a conspicuous need for further effort is clear. None of the risk factors encountered in our review existed in our patient, yet the devastating complication of quadriplegia occurred, which was much more extensive than expected and described previously in the literature. The literature is lacking when it comes to managing BAI in an emergent situation and the consideration for revascularization in such settings. We conclude by reiterating and echoing the appeal for stronger evidence-based guidelines in TEVAR used to manage BAI.
Footnotes
Disclosure
The authors declare no conflict of interests, support, or funding from any pharmaceutical company.
- Received May 21, 2014.
- Accepted October 13, 2014.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.