Abstract
Headache is a potential complication of epidural injection. We report a patient who developed headache 5 days after a lumbar epidural steroid injection, which was not related to the epidural procedure, but caused by Duloxetine induced hyponatremia. Antidepressant drug induced headache should be considered in the differential diagnosis of post dural puncture headache.
Multimodal analgesic therapy, which includes antiepileptic and antidepressant drugs, is commonly used to control chronic low back pain. In addition, interventions, such as lumbar epidural steroid injection (LESI) are offered to some patients. Headache after the epidural injection is a known potential complication, which is usually due to dural tap. This headache has its characteristic symptoms related to posture. When the headache after the epidural injection is atypical, other causes should be considered in the differential diagnosis. We present a case where the patient developed headache after the LESI not due to the epidural injection, but due to Duloxetine induced hyponatremia. Our objective in presenting this particular case is to highlight that antidepressant drugs should be considered in the differential diagnosis of post dural puncture headache.
Case Report
A 74-year-old woman presented to the pain clinic with a history of chronic left sided sciatic pain that also restricted her mobility. This pain was worse at night and affected her sleep. She had been treated with nonsteroidal antiinflammatory drugs, pregabalin, tramadol, and acetaminophen without much relief. In addition, she was taking beta and angiotensin receptor blockers and aspirin. On examination, this slim pleasant lady had limited left leg raising with tenderness in her lower back. After the death of her husband, she has lived with her daughter, and she has a good memory. Her lumbar spine MRI revealed multilevel disc prolapse and spinal canal stenosis. The routine laboratory results (blood cell count, urea and electrolytes, and clotting studies) were within normal limits. In the clinic, after discussion with the patient, we decided to perform LESI the next week, and Duloxetine 60 mg/day was prescribed. Taking full aseptic precautions, an LESI was performed using a Portex™ epidural set under C-arm fluoroscopy. The correct placement of the epidural needle was confirmed by an epidurogram with the radio-contrast (Omnipaque™), after which 10 ml of 0.9% normal saline (NS) containing 80 mg methyl prednisolone was injected into the epidural space. After the procedure, she was observed in the recovery room for an hour and then discharged home and advised to continue her medication and return to the pain clinic after 4 weeks. Three days after the LESI, she was contacted on the phone to inquire about her wellbeing, and she reported some reduction in pain and was able to sleep. Three days later, she came to the hospital in the morning, with a 24 hour history of severe headache and being unable to sleep despite extra doses of acetaminophen. Along with the headache, she also complained of persistent nausea and one episode of vomiting. The headache was continuous, not throbbing in nature, felt over her occiput, both temples, vertex, and forehead and not associated with neck pain or stiffness. The pain was relieved a bit in the sitting position. With a normal body temperature, she was alert, well orientated, a bit restless with no neurological deficit on examination. Except for some puffiness around her eyes, her clinical examination was normal and she appeared to be euvolemic. She denied any change in her urinary or bowel habit. A brain CT scan was unremarkable. Her blood tests revealed severe hyponatremia (sodium 112 mmol/L, normal range: 135-145 mmol/L) with low serum osmolality of 248 mosmol/Kg (normal range 280-295). The remaining electrolytes, urea, creatinine, and blood counts were within normal limits, and her urine osmolality was 328 mosmol/Kg.
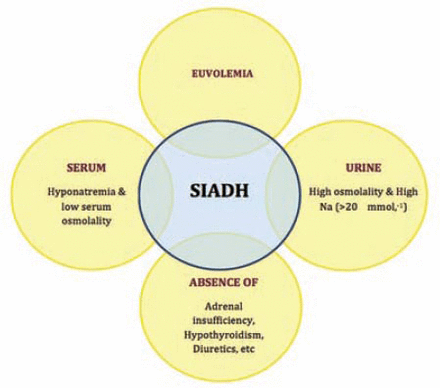
She was diagnosed to have Duloxetine induced syndrome of inappropriate antidiuretic hormone secretion (SIADH) causing severe hyponatremia (Figure 1). Her treatment started with intravenous (IV) acetaminophen 1 gm and granisetron 1 mg and IV infusion of 3% hypertonic saline commenced at the rate of 50 ml/hour. Eight hours later, her headache was still bad but the nausea improved, and serum sodium improved to 118 mmol/L. She then received codeine 30 mg intramuscular along with a second 1 gm dose of IV acetaminophen. One hour later she went to sleep in a semi sitting position. Her vital signs remained normal. After 3 hours she woke up. The headache was there but better than before. Eighteen hours after her hospital admission, the serum sodium further improved to 122 mmol/L. Hypertonic saline infusion was then replaced with NS. The headache was mild with no nausea or vomiting. She had a bowl of soup, she took her daily medications except Duloxetine and slept for another few hours. The next morning, 30 hours after her admission, she described her headache as heaviness instead of pain. With a serum sodium of 128 mmol/L, the IV infusion of NS was discontinued. She was kept under observations for another 36 hours, when her headache completely resolved, and with serum sodium at 130 mmol/L, she was discharged home.
Diagnostic criteria for the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Na - sodium
Discussion
Headache is high on the list of almost all informed consents taken for epidural injection.1 The most common cause of headache after epidural injection is an inadvertent dural puncture. This headache, also called post dural puncture headache (PDPH) is due to CSF leakage, and low intracranial pressure. This typically presents as a postural headache that is aggravated in the sitting and standing position and relieved by lying down. A PDPH may occur up to 5 days after the procedure is performed.2 Any atypical headache after an epidural procedure should be taken seriously and its cause should be identified (Table 1).
Differential diagnosis of post epidural injection headache.
The headache in our patient presented 5 days after having an epidural injection. Clinically, this headache did not mimic headaches that develop as a complication of the epidural injection, such as PDPH or meningitis. The only positive laboratory finding was hyponatremia that caused low serum osmolality. The pre-procedure urea/electrolytes were within normal limits. The probable diagnosis of SIADH was based on euvolemia, hyponatremia with low serum and high urine osmolality, and Duloxetine appeared to be the most likely cause of SIADH in our patient.
Duloxetine is a serotonin-norepinephrine reuptake inhibitor (SNRI), which was introduced a decade ago in North America for the treatment of major psychiatric disorders.3 Later its indications expanded to include various painful conditions such as neuropathic pain and fibromyalgia.3 Maramattom in 20064 reported the first case of Duloxetine induced SIADH in a woman, where the drug was used as an antidepressant. Thereafter many cases of Duloxetine induced SIADH have been reported in the literature.5-8 Though hyponatremia is a rare side effect of Duloxetine, elderly females with a low body weight appear to be more susceptible.7 Duloxetine is a relatively new drug in the management of chronic pain, and appears to be a cost-effective post-first-line treatment for chronic low back pain.9 Worldwide, the use of SNRI has increasingly become common in chronic pain patients, especially in patients with diabetes with painful neuropathy and sciatica. Because of the risk of developing SIADH, it would be advisable to monitor serum sodium in patients who are taking SNRIs.
Fortunately, our patient, apart from headache and nausea did not have any other CNS symptoms (lethargy, disorientation, convulsion, coma). The SIADH can cause brain edema, an increase in intra cranial pressure, and may lead to brain stem herniation.10 There was clinical suspicion of raised intracranial pressure in our patient, because of pain relief in the sitting position and as the headache was associated with nausea and vomiting, we decided not to perform a diagnostic lumbar puncture. Symptomatic hyponatremia is a medical emergency and we started its treatment immediately with 3% hypertonic saline with a goal of slowly raising the serum sodium concentration, not more than 5 mmol/L per 12 hours. Rapid correction of hyponatremia may seriously harm the patient by causing demyelination of neurons.
In conclusion, our case is that of post epidural injection headache not caused by the epidural procedure per se, but coincidently caused by a Duloxetine induced hyponatremia.
CASE REPORTS
Case reports will only be considered for unusual topics that add something new to the literature. All Case Reports should include at least one figure. Written informed consent for publication must accompany any photograph in which the subject can be identified. Figures should be submitted with a 300 dpi resolution when submitting electronically or printed on high-contrast glossy paper when submitting print copies. The abstract should be unstructured, and the introductory section should always include the objective and reason why the author is presenting this particular case. References should be up to date, preferably not exceeding 15.
Acknowledgments
We thank Dr. Taqi Khan, Kidney Transplant Surgeon at Prince Salman Armed Forces hospital, Tabuk, Saudi Arabia for his expert advice in the preparation of this manuscript.
- Received December 15, 2014.
- Accepted March 16, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.