Mirror movements (MM) are involuntary movements which occur on one side of the body as a mirror reversal of the intentional movement on the other side of the body. Mirror movements, which are normally observed during early childhood, decrease with development of the brain, and then usually disappear around the end of the first decade of life. Persistence of MM in adulthood is considered pathological and can be seen in various congenital brain disorders. Severe MM of the hands can hinder the activities of daily living which require bilateral hand coordination. However, little is known on the management modality of MM.1,2 The pathogenetic mechanism of MM has not been clearly elucidated; however, the most plausible mechanism is the ipsilateral motor pathway, which involves from the unaffected motor cortex to the affected limb based on the inhibition hypothesis.3 Many studies have demonstrated the safety and effectiveness of repetitive transcranial magnetic stimulation (rTMS) for suppressing the unaffected motor cortex in stroke patients. In addition, a recent study demonstrated the cessation of MM and deactivation of the unaffected motor cortex by rTMS for 2 weeks in a patient with a cerebral infarct.2 Regarding MM in patients with a congenital brain lesion, one study reported that rTMS for 2 weeks resulted in relief of MM in a patient with congenital MM.1 However, no functional neuroimaging study on the effect of rTMS on the unaffected motor cortex in a patient with a congenital brain lesion has been reported. In this study, we report on a patient with porencephaly in whom the intensity of MM and activation of the unaffected motor cortex were decreased after application of rTMS, as evaluated by functional magnetic resonance imaging (fMRI).
A 21-year-old male patient had exhibited left hemiparesis since birth. He was delivered at full-term without complications. He was diagnosed as a congenital porencephalic cyst in the right frontal area and underwent shunt operation at 6 months after birth. He complained of severe MM, and as a result, performing bimanual activities such as typing is difficult. He signed an informed consent statement, and the study protocol was approved by the Institutional Review Board of a university hospital.
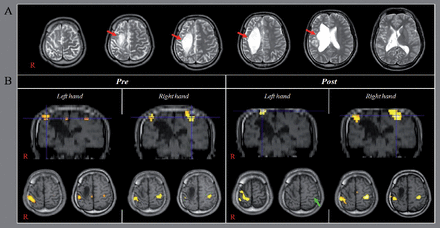
Frameless stereotaxic neuronavigation (TMS-Navigator, Localite, Sankt Augustin, Germany) based on the coregistered patient’s T1-weighted image was used for navigation of the TMS coil and to maintain its precise location and orientation throughout TMS sessions. The patient underwent 10 sessions of rTMS performed to the activation area of the primary sensori-motor cortex (SM1) in the left hemisphere on fMRI, according to the following protocol: frequency - 1 Hz, intensity of motor threshold - 100%, 1200 stimuli as a single, continuous train lasting 5 minutes, for 5 sessions per week for a period of 2 weeks. Mirror movements were evaluated according to the degree of involuntary movement of the unaffected hand during performance of a functional MRI task using the modified MM scale by Woods and Teuber (1978).4 The Purdue Pegboard test was used for evaluation of hand dexterity of both hands. Blood oxygenation level dependent (BOLD) fMRI measurements were performed 2 times (pre-rTMS and post-rTMS after 2 weeks), using a 1.5-T. Using a block paradigm (21s control, 21s stimulation: 3 cycles), hand grasp-release movements (1Hz) were performed for stimulation. SPM 8 software (Wellcome Department of Cognitive Neurology, London, UK) running in the MATLAB environment (The Mathworks, Natick, Mass., USA) was used for analysis of fMRI data. For changes in BOLD signal, the control condition data were subtracted from the stimulated condition data. Statistical parametric maps were obtained, and voxels of cluster level were considered significant at a threshold of uncorrected p<0.001. On pre-rTMS fMRI, movement of fingers on one hand resulted in a grade 4 MM of the fingers of the opposite hand (the movement range of the metacarpophalangeal (MP) joint: the right hand- 50°, and the left hand- 60°). By contrast, on post-rTMS fMRI, movement of fingers on one hand on the first fMRI resulted in a grade 3 MM of the fingers of the opposite hand (the movement range of the MP joint: the right hand- 10°, and the left hand- 20°). The Purdue Pegboard score (PPC) for both hands was increased after rTMS (the right hand-from 14 to 16, and the left hand- from 2 to 4). On pre-rTMS fMRI, bilateral primary SM1s were activated during movements of either hand (Figure 1). Activation of the left SM1 was no longer observed on post-rTMS fMRI during movements of the left hand and bilateral SM1 were activated during movements of the right hand. In the current study, we followed up the change of MM and fMRI in a patient with congenital MM. According to our results, it appeared that the MM of both hands was decreased along with the deactivation of the left primary motor cortex. In addition, the fine motor activity of both hands was increased. As a result, rTMS for 2 weeks resulted in improvement of MM and fine motor skill of both hands. These results coincided with those of the previous study conducted for 2-week’s rTMS in a patient with congenital MM.1 The basic mechanism of the disappearance of MM at or around 10 years after birth has been explained by the inhibition hypothesis;3 Maturation of a callosally mediated 2-way inhibitory system through which each hemisphere supresses the ipsilateral corticospinal tract of the contralateral hemisphere.1,2 Consequently, the time of disappearance of MM at the approximate age of 10 years is known to generally coincide with the time of maturation of the corpus callosum region for transcallosal motor fibers and the disappearance of the ipsilateral corticospinal tract.3
Results of functional MRI A) Brain MR images showing a large cyst in the right frontal lobe by a radiologist (Woo Mok Byun) (red arrows). B) On pre-repetitive transcranial magnetic stimulation (TMS) functional MRI, bilateral primary sensori-motor cortices (SM1s) are activated during movements of either hand (B). Activation of the left SM1 (green arrow) is no longer observed on post-repetitive TMS functional MRI during movements of the left hand and bilateral SM1 are activated during movements of the right hand.
For the past 2 decades, many studies have used rTMS to suppress activation of the ipsilateral motor pathway from the unaffected cortex to the affected hand in stroke patients. However, only a few studies have reported that rTMS was effective for control of MM.1,2 Kim et al1 reported that MM was decreased by 2-week’s rTMS in an 8-year-old boy with congenital MM, his MM decreased from G4 to G1 in both hands with improvement of fine motor skill and this decrement of MM was maintained until 18 months after 2-week’s rTMS.1 In a recent study, Seo and Jang2 who applied 12 sessions of r-TMS for 2 weeks on the unaffected motor cortex in a patient with cerebral infarct, observed the disappearance of MM in the affected hand and activation of the unaffected motor cortex concurrent with motor recovery of the affected hand.
In conclusion, the decrement of MM and activation of the unaffected motor cortex by 2-week’s rTMS was demonstrated in a patient with porencephaly. We believe that our results have important implications in rehabilitation for patients with congenital brain lesion who have suffered from severe MM. However, this study is limited by the fact that it is a case report. Conduct of further complementary studies involving larger numbers of cases is warranted.
Acknowledgment
The authors gratefully acknowledge the National Research Foundation of Korea (NRF) for supporting this study.
Footnotes
Disclosure
This work was funded by the Korea government National Research Fund grant no 2015R1D1A4A01020385.
- Received October 21, 2015.
- Accepted January 20, 2016.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.