Abstract
Objective: To evaluate the efficacy and safety of levetiracetam (LEV) in the management of seizures in neonates.
Methods: A prospective non-blind, single arm clinical trial conducted in the Department of Neonatology and Pediatric Intensive Care, Mohamad Kermanshahi, and Imam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran from May 2014 to December 2014. Fifty out of 60 newborns with gestational age ≥30 weeks with clinically diagnosed seizures were included. Levetiracetam was administered orally with an initial dose of 10 mg/kg twice a day. The patients were observed continuously by Neuro Intensive Care nurses, and visited daily by a neuropediatrician in the first 7 days and then at days 14, 30, and 90 after the start of LEV administration. Clinical examination was performed for every patient, and seizure number, antiepileptic medication, and adverse events were detailed at every visit.
Results: 47 infants were seizure free under LEV at the end of the first week, 47remained seizure free at 4 weeks, and 46 remained seizure free at 11 weeks. No immediate and long-term side effects were noted in our patients.
Conclusion: This study investigated the efficacy and safety of LEV in neonatal seizure control but confirmation with further randomized controlled trials is required.
Neonatal seizures have undesirable effects on brain development, so their early recognition, and treatment is critical to prevent mortality and future motor or cognitive disability.1-3 The incidence of seizures in the neonatal period is 5 per 1000 live births, and in preterm neonates is 11 per 1000 live births.1,2 At present, phenobarbital (PB) remains the first-line treatment for neonatal seizures.4 Efficacy of traditional treatment (PB and phenytoin) in neonates is 30-50% for stopping of electrical seizures.5 Additionally, there is increasing concern related to the long term adverse effects of PB, it was shown to increase neuronal apoptosis in animal studies6 and leads to cognitive impairment in children.7 Levetiracetam (LEV) is a second-generation anticonvulsant drug that since 2004 has been approved by the FDA as an add-on to other antiepileptic medicines for partial onset seizures treatment in children 4 years of age and older, and again, in 2012, for adjunctive therapy in partial onset seizure in adults and children one month of age and older with epilepsy, but not for neonatal seizures.8 In contrast to most other established antiepileptic drugs, LEV was found not to have neurodegenerative effects in early animal studies,6 and it is considered safe because of linear pharmacokinetics, fast and approximately full absorption after oral intake, slight protein binding, mainly renal elimination, and no established serious drug interactions.9 Therefore, the present study evaluated the efficacy and safety of LEV in the management of seizures in neonates.
Methods
Search method
From 2009 to 2014, we searched MEDLINE, Ovid, CINAHL, EBSCO, and PubMed for full-text articles written in English with proper subject terms: 1) neonatal seizure; 2) treatment; and 3) levetiracetam.
Study design
The study was performed as a prospective non-blind, single arm clinical trial from May 2014 to December 2014. Our subjects were chosen from neonates with the diagnosis of seizure admitted to the Department of Neonatology and Pediatric Intensive Care of Mohamad Kermanshahi and Imam Reza Hospital affiliated to Kermanshah University of Medical Sciences, Kermanshah, Iran. The diagnosis of seizure was established clinically, and no continuous EEG monitoring was performed at the time of diagnosis. The local ethics committee approved the study protocol, and written informed consent was obtained from the parents of newborns. This study was carried out according to the principles of the Helsinki Declaration. The patients were included based on the following inclusion and exclusion criteria.
Inclusion criteria
1. Gestational age ≥30 weeks; 2. Postnatal age ≤29 days; 3. Birth weight ≥2000 grams; 4. Clinical seizures requiring treatment with an antiepileptic medication (as per the judgment of the clinician caring for the patient)
Exclusion criteria
1. Neonates with renal insufficiency indicated by serum creatinine ≥1.; 2. Neonates whose seizures were caused by electrolyte disturbances or hypoglycemia, or whose seizures in response to pyridoxine.
Neonatal seizures were defined according to Volpe’s classification10 as subtle, focal, clonic, multifocal clonic, focal tonic, generalized tonic, and myoclonic. Because LEV (Keppra, UCB Pharma S.A. Brussels, Belgium) intravenous (IV) formulation were not available at the beginning of the study; we used Keppra oral solution. Levetiracetam was administered as the first-line therapy and intravenous PB and phenytoin were allowed as the second and third–line therapy only during the first 2 days of LEV titration. Levetiracetam was administered orally with an initial dose of 10 mg/kg twice a day. The daily dose was allowed to be increased by 10 mg/kg over 3 days up to 30 mg/kg, depending upon the clinical response. This dosage protocol was selected according to pervious study.11 The patients were observed continuously by Neuro Intensive Care nurses and visited daily by a neuropediatrician in the first 7 days and then at days 14, 30, and 90 after the start of LEV administration. Clinical examination was performed for every patient, and seizure number, antiepileptic medication, and adverse events were detailed at every visit. The EEG was performed during the first 7 days after drug initiation and at the end of the first month and at 3 months. After one week of seizure control, decisions regarding further treatment were considered individually.
Cerebral ultrasound was performed in all infants in the first 48 hours. Laboratory tests including complete blood count, hepatic and renal function parameters were performed before treatment initiation, and at the end of the first week and at all further visits. The LEV serum levels were not measured.
Statistical analysis
Patient’s demographic and clinical information were recorded in a predesigned checklist. The data were analyzed with the Statistical Package for Social Sciences software version 19 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to report variables. Values were expressed as means±SD or as percentages.
Results
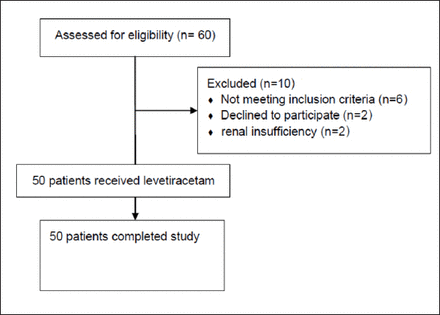
Fifty of 60 newborns with clinically diagnosed seizures admitted to the emergency room were included. A flow chart of patient enrollment and disposition is provided in Figure 1. The mean age was 8.7 days, the gestational ages ranged from 30-41 weeks and birth weights between 2050 to 4200 kg. The demographic and clinical characteristics of all infants included in the study are summarized in Table 1.
Consort flowchart of patient enrollment and disposition.
Demographic and clinical characteristics of 50 newborns clinically diagnosed with seizures in Iran.
Three newborns suffered status epilepticus. All other newborns presented with repetitive seizures. Forty-seven newborns receiving LEV became seizure free within a few days at LEV doses of 20 mg/kg/day. Others needed to LEV dosage to 40 mg/kg/day.40 Forty newborns required an additional use of PB 20 mg IV as acute management in the first 24 hours after the start of LEV treatment, because of seizure repetition during LEV titration. Out of 40 patients, 10 patients received an additional dose of PB 20 mg IV on the second day after treatment initiation and 3 of whom acquired additional treatment with phenytoin. Forty-seven neonates were seizure free under LEV at the end of the first week, and 47 remained seizure free after 4 weeks, and 46 remained seizure free after 12 weeks, while EEG markedly improved in 10 patients at the end of the fourth weeks. Three infants had seizure recurrence during the first week, in all of whom LEV substituted with PB. In 25/50 cases, LEV was discontinued after 2-4 weeks of seizure freedom. Levetiracetam was continued in 25 cases up to 3 months after treatment initiation. No immediate and long-term side effects were noted in our patients.
Discussion
Our study revealed that LEV was effective in controlling neonatal seizures in 47 patients. Our results are in agreement with data from randomized trials conducted by Khan et al.12 They retrospectively studied 22 term neonates who were treated with intravenous LEV as a second-line drug after PB therapy failure, and revealed that seizure was terminated in 86% (19/22) of subjects one hour after the initial dose. All patients were seizure-free by 72 hours.12 In other study conducted by Khan et al13 12 preterm neonates with seizures who received levetiracetam were studied.13 Their results showed that 10 patients achieved seizure cessation by 72 hours and at 6 months follow up, 6 were seizure free and were weaned off medication and 3 remained seizure free on oral medicine. Abend et al14 in a retrospective cohort study, reviewed 23 neonates with EEG-confirmed seizures who received LEV. They observed LEV was associated with a greater than 50% seizure reduction in 35% (8 of 23), including seizure termination in 14 (30%). Four out of 23 neonates in this trial, received LEV as first-line therapy, and 14 patients received LEV as second-line after PB and the remaining 5 patients received LEV as third-line or later. In a prospective feasibility study carried by Ramantani et al.11 Intravenous LEV was applied as first-line treatment in 38 preterm and term newborns. Their study revealed that LEV is safe and effective in controlling neonatal seizures,11 Furwentsches et al15 studied prospectively the use of oral LEV in 6 newborns (gestational age 31-41 weeks). In their study all 6 newborns were seizure-free within 6 days and 4 of the 6 remaining seizure-free at 3 months.15
No serious adverse effects were seen in our patients. These results were consistent with findings of most of the previous studies.11-16 The 47 newborns receiving LEV became seizure free within a few days at LEV doses of 20 mg/kg/ day. Different studies have described a wide broad range for LEV dosage ranging from 10 to 80 mg/kg/day. Merhar et al17 studied the pharmacokinetics prospectively of LEV in 18 newborns with seizures. They pointed that neonates had lower plasma clearance, higher volume of distribution, and longer half-life compared with older children and adults. They suggest that due to the higher volume of distribution in newborns, the loading dose should be higher compared to older children and adults, and on the basis of lower plasma clearance, the dosing interval may need to be increased. So the appropriate dosage of LEV in neonates needs to be studied further.
There were some limitations to this study. First, continuous EEG monitoring before and during the first week of treatment was not performed, and the evaluation of seizure response was based on clinical observations. Seizures in neonates are distinct from those of older children and adults. The most common neonatal seizures are defined as subtle because the clinical presentations are often missed. Second, the lack of control group, third, the small population size, and finally, blood levels of antiepileptic drugs were not measured.
In conclusion, this study illustrated the efficacy and safety of LEV in neonatal seizure control, but confirmation with further randomized controlled trials is required.
Footnotes
Disclosure
The authors have not disclosed any affiliation or financial involvement with organizations or entities with a direct financial interest in the subject matter or materials discussed in the manuscript. The study was registered in the Iranian Registry of Clinical Trials (www.irct.ir) with registration number ID: IRCT2014070318334N1
- Received November 25, 2015.
- Accepted March 9, 2016.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.