Abstract
Objectives: To evaluate the effectiveness of an early mobility protocol for stroke patients in the intensive care unit.
Methods: Participants were patients with first or recurrent stroke (n=60, age=49.02±6.36 years, body mass index=32.95±5.67 kg/m2) admitted to the intensive care stroke unit in general hospitals, Riyadh during October and December 2016. Single group pretest-posttest design involving an early mobility protocol was started within first 24 hours admission. Pre and post measurements of muscle strength, pulmonary function and quality of life were carried out.
Results: There were significant improvements in muscle strength of upper and lower extremities´ muscles after treatment (p<0.05), pulmonary functions including Forced Vital Capacity, Forced Expiratory Volume 1 (p<0.05) and quality of life, namely, Barthel Index and modified Rankin Scale (p<0.01).
Conclusion: This study demonstrates that initiating an early mobility protocol is safe and effective for intensive care unit stroke patients and supports introducing the current protocol as a standard protocol in neurogenic Intensive Care Units.
Stroke is a life-threatening condition caused by interruption of the blood supply to any part of the brain. Stroke causes acute neurological disorders and long-term disabilities and imposes economic, social and health impacts on individuals and their families.1 Survivors of stroke are left with mental and physical disabilities that cause social and economic burdens and impair quality of life (QOL). In Saudi Arabia stroke is becoming a rapidly increasing problem and a primary cause of morbidity and mortality.2 Worldwide the incidence of first-time stroke was 17 million during 1990-2000.3 Cerebrovascular diseases including stroke is a leading cause of mortality,4 and stroke is the fifth leading cause of death, but it remains the first cause of disability in the USA.5 By 2030 there will be almost 12 million stroke deaths and 70 million stroke survivors globally.6 Stroke has an adverse influence on the QOL of patients. The onset of stroke is sudden, and unlike other disabling conditions, it leaves patients and their family’s ill prepared for its sequelae.7 Stroke may create unique conditions that affect the patients’ QOL, involving dysfunctions in physical, emotional, memory, thinking, and social interactions.8
Stroke is an urgent health care issue. It is a common cause of the hospital admissions. Immediate admission to the neuro-intensive care unit can facilitate early stroke treatment strategies.9 Stroke patients in the intensive care unit (ICU) experience a decrease in physical activity that represents a significant stress on the body and leads to a considerable decrease in functional status, increases morbidity, mortality rate, and duration of hospital stay and cost of care.10 In addition to comorbid diseases, patients on mechanical ventilation have many barriers to mobility because they are surrounded by tubes, catheters, life support and monitoring equipment. Additionally, other factors besides weakness, such as sleep loss, lack of social communication, nutritional status, sedation, and an ICU culture that encourages bed rest further contribute to functional deterioration.11 There is considerable loss of the muscle mass during the initial weeks of immobility in the ICU, therefore its management is inherently related to QOL after discharge.12 Considerable published evidence indicates that patients in ICUs have high morbidity and mortality, high costs of care and a marked decline in functional status.13,14
Early and progressive mobilization program has been described as a key component for patients in the ICU. It may decrease post stroke complications such as infections, deep venous thrombosis, pneumonia, pressure ulcers, falls and de-conditioning with bed rest.15 It has been recognized that mobilization of post stroke patients is essential to prevent hospital-associated complications, functional decline and facilitate recovery.16 Moreover, the benefits of early mobilization include decreased ICU-acquired weakness, improved functional recovery within hospital,17 Effective stroke intervention begins the day the patient has a stroke.18 It has a positive effect on patient functional ability, promotes positive psychological effects and improves walking at hospital discharge and reduces hospital length of stay.19 While on the other hand, long term inactivity may affect the patients’ physical, social, emotional, behavioral, and psychological pattern.20 In addition, secondary changes associated with stroke-related inactivity include muscle atrophy, a shift in muscle fiber type to a greater predominance of fast-fatigable, insulin-resistant fibers, loss of cardiovascular fitness, and increased intramuscular fat.21 Therefore, early mobilization program which is a complex intervention that needs crucial patient assessment and management, as well as interdisciplinary team collaboration and training.22,23 The early mobilization may improve patient outcomes and recovery.24 Few studies have investigated the role of increased mobility in ICU patients. Therefore, this prospective intervention trial evaluated the effectiveness of an early mobility program administered by physical therapists and nursing personnel for stroke patients admitted in ICU.
Methods
Study design
A single group pretest-posttest design was used.
Setting and sample
Seventy patients were screened for eligibility, of which 62 fulfilled the inclusion criteria, 2 patients died and their data were not used in the statistical analysis. Sixty patients with confirmed stroke (infarct or intracerebral hemorrhage) who were admitted to the ICUs in general hospitals in Riyadh, Kingdom of Saudi Arabia within 24 hours of stroke onset participated in this study, during October and December 2016. Patient inclusion criteria included an age of 40-60 years; because the muscle strength is affected by age, admitted to the ICU within 24 hours and without lower limb motion limitations. Exclusion criteria were hemodynamic instability, and any muscle weakness. The contraindications for initiating early mobility were, mean arterial pressure less than 65 mmHg, heart rate less than 60/min or above 120/min, respiratory rate less 10/min or above 32/min, and pulse oximetry less than 90%, insecure airway device or difficult airway, falls, removal of endotracheal tubes (ETT), removal or dysfunction of intravascular catheters, removal of catheters or tubes and cardiac arrest.
Ethical considerations
The Declaration of Helsinki principles were followed in this research; the ethical approval was obtained under the number 64/52362, 21/12/1437. Informed consent was obtained from all patients or their nominated representatives.
Measurements/Instruments. Pulmonary functions tests (PFTs)
Pulmonary function tests are a group of tests that evaluate the capacity of the lung.25 Zan-680 Ergospiro Ergospirometry System was used to measure pulmonary functions; Forced Vital Capacity (FVC) and Forced Expiratory Volume 1 (FEV1). These tests were carried out after cessation of a mechanical ventilator. Measurements were repeated after ten days of treatment.
Measurement of muscle strength
A hand-held dynamometer, small enough to hold in one hand and quickly read, was determined to be sensitive in detecting muscle strength reduction in the critically ill. The device had a liquid crystal display screen and 6 function buttons that controlled the menus and allowed option selection.26 Patients were instructed to apply maximum isometric contraction during dynamometry measurement. The dynamometer was placed perpendicular to the limb being measured. Biceps brachii, triceps, deltoid muscles in upper extremity and quadriceps, dorsiflexors, and plantar flexor muscles in lower extremities were evaluated bilaterally.23 The measurement was carried out before and after 10 days of treatment. The same examiner repeated the procedure and patients would rest for 1 minute between 2 consecutive trials. Patients tried 3 times for each muscle, and the best reading among 3 trials was taken. Minimal change in muscle strength was measured in units of weight such as pounds or kilograms.27
Quality of life measures. Barthel Index
Barthel Index is scale used to evaluate patient activity or recovery.15 It’s score ranges from 1 to 100. Patients were categorized by the following grades: 1) mildly recovered; score between 0-30; 2) moderately recovered; score between 31 and 60; and, 3) fully recovered; patients who scored between 61 and 100.
The modified Rankin Scale
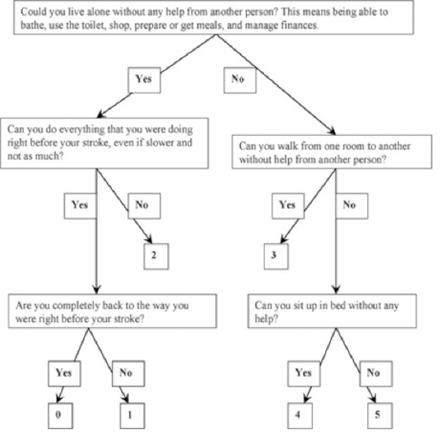
The modified Rankin Scale is an ordinal scale ranging from zero (no disability) to 5 (severe disability), with a score of 6 allocated to those who die. A favorable outcome is defined as modified Rankin Scale scores of 0-2 (no or minimum disability), and poor outcomes were considered as scores of 3-6 (moderate or severe disability, or death.28 The scoring is illustrated in Figure 1.
Progression of patients through the study.
Data collection/Procedure
Before each intervention, a nurse discussed the patient’s medical status and provided necessary medication and support for patient safety. A quick evaluation was carried out by a physical therapist before each physical therapy session to determine which category (Table 1) was appropriate for the patient’s condition.
Categories of stroke patients.
All procedures were briefly described to patients, according to their level of consciousness, before the treatment protocol began. All techniques were conducted under the supervision of researchers. The protocol was stopped if any sign of hemodynamic instability was seen. Vital functions were regularly monitored during mobility activity. Therefore, a coordinated collaboration among members of the multidisciplinary health care team ensured proper care for and patient safety.29
Treatment modalities were administered depending on the stage of illness (patients were classified into 3 categories) (Table 1). Each session was of 60 to 90 minutes according to patient’s category. For category 1: 50 min of transcutaneous electrical muscle stimulation, 15 minutes of passive proprioceptive neuromuscular stimulation, 15 minutes of chest therapy and 5 to 10 minutes change position every 2 hours. For category 2: 30 minutes of transcutaneous electrical muscle stimulation, 15 minutes of chest therapy and 15 minutes of active exercises. For category 3: 15 minutes of chest therapy and 45 minutes of mobilizing and resistance exercises, as shown in Table 1.
Category 1
Acutely ill, unstable, requires life-support equipment such as mechanical ventilator. Modalities applied were passive proprioceptive neuromuscular facilitation, positioning, and splinting, transcutaneous electrical muscle stimulation, rolling side to side, and sitting balance. Application of these modalities required minimum level of patient cooperation, and involved minimal stress on the cardiorespiratory system.
Category 2
Stable cooperative patient but still on mechanical ventilation. Modalities applied were transferring patient, resistive muscle training, active walking with and without assistance.
Category 3
Cooperative patient, and weaning from mechanical ventilation. Modalities applied were resistive muscle training using pulleys, elastic bands, and weight belts, walking, deep breathing exercises, interventions to increase expiratory flow such as huffing and coughing, manually assisted cough.
Modalities descriptions. Proprioceptive neuromuscular facilitation
Proprioceptive neuromuscular facilitation was administered in the form of passive diagonal movement. Stretching techniques were applied for both upper and lower extremities. The frequency of Proprioceptive neuromuscular facilitation was 2 sets of 10 repetitions per each diagonal movement bilaterally.30
Positioning
The patient was positioned upright, supported to prevent soft tissue contractures, protect flaccid limbs, and avoid nerve compression, and skin breakdown. The rotations were carried out every 2 hours.31
Transcutaneous electrical muscle stimulation
Transcutaneous electrical muscle stimulation is a modality used to maintain muscle mass and strength in different conditions particularly in the ICU setting due to rapid loss of muscle mass. It is also used for immobile sedated patients. It was applied for 55 minutes, daily for the muscles of the lower extremity.32
Transfer & mobility technique
Early transfer and mobility techniques were started as soon as the patient’s medical condition was stable. For patients unable to ambulate, who have limiting weakness but can usually tolerate bed activities, therapeutic exercises were started. Then, they progressed to turn from side to side and sit on the edge of the bed. Trunk control was stimulated by applying sitting balance activities. When limb and trunk muscles strengthened, standing with a walker or assistance began. At first, standing was for short periods and then gradually increased. The patient was transferred to a chair and, time in the chair was increased gradually. Walking activities were started in place, i.e., weight shifting, and stepping in place. Walking re-education was encouraged when appropriate; distance and duration depended on the patient’s tolerance. The intervention was performed, once a day for 10 days or until patient discharged.33,34
Chest physical therapy
Chest or respiratory physical therapy was introduced to patients in the ICU to improve respiratory status. Chest physical therapy included percussion; vibration; suction; cough, and breathing exercises. Techniques were applied to increase inspiratory volume including deep breathing exercises, mobilization, incentive spirometry, and body positioning. Techniques were performed to increase expiratory flow such as huffing and coughing, and manually assisted cough.35
Data analysis
Statistical analysis was carried out using statistical package for the social sciences; SPSS Inc., Chicago, IL, USA, version 22. Differences were assumed significant at p-value <0.05. Continuous variables were described in terms of mean and standard deviation while categorical variables were described by frequencies (number of cases), and relative frequencies (percentages). Paired T-test was used to test the differences in (muscle strength, forced vital capacity, forced expiratory volume 1) between pre and post treatment. Wilcoxon signed-rank test was used to test the differences in QOL measures between pre and post treatment.
Results
Table 2 describes the patient characteristics. The mean age for the studied sample was 49.02±6.36 years with a range of 40 to 60, while the body mass index ranged between 21.4 and 44.0 kg/m2. The duration of stroke and ICU stay had a range of 3 to 24 hours and 2 to 15 days, respectively. Majority of the participants (63.3%) were male. Large infarction stroke were the most numerous group. Almost half of the patients (28 out of 60) had diabetes, or hypertension with diabetes or ischemic heart disease.
Descriptive Statistics of the study participating stroke patients. N=60
Outcome measures
Muscle strength and pulmonary functions before and after the treatment. The mean values of muscle strength of biceps, triceps, deltoid, quadriceps, dorsiflexors, planter flexors for right (RT) and left (LT) sides before and after treatmentare shown in Table 3. Muscle strength and pulmonary function significantly increased after treatment.
Pre-post clinical measurements: muscle strength, pulmonary functions, and quality of life. N=60
Quality of Life Measures. Barthel Index (BI) before and after the treatment
Table 3 shows median scores of BI before and after treatment. The median score value increased from 15.00 before treatment to 42.00 after treatment; patient´s level of activity progressed from mild to moderate level (p=0.001).
Modified Rankin grade before and after the treatment
Table 3 shows frequency and percentage of modified Rankin grades before and after treatment. Before treatment, about 34 (56.7%) were considered grade 5 and 26 (43.3%) were grade 4. After treatment, patients’ QOL improved because only 24 (40.0%) were considered grade 4; the majority of patients 36 (60.0%) progressed to grade 3 (p=0.001).
Discussion
The coordinated management of stroke patients in the ICU using physical modalities, mobilization, and exercises involving crucial role of nurses and physical therapists helped in increasing their muscle strength, pulmonary function and quality of life. Early mobilization protocol is considered extremely important for the patient in the ICU. It has a positive effect on patient functional ability and reduces ICU and the overall duration of hospital stay.22,36 The finding of this study further reinforces the positive impact of the early mobilization protocol for stroke patients in the ICU.
The finding of significant improvements in muscle strength of the upper and lower extremities after treatment is similar to previous studies.37 High-intensity exercises such as resisted exercises (eccentric, concentric, and isometric exercises) that are carried out against manual or mechanical resistance from lying position do not counteract the adverse effects of prolonged bed rest because intravascular fluids shift away from the extremities to the thoracic cavity because of lack of gravitational stress.37 Therefore, upright positioning helps maintain optimal fluid distribution and improves orthostatic tolerance. Many of the survivors could ambulate more than 100 feet (30 meters) at the time of discharge with the use of the early activity protocol.38
The finding of significant improvements in pulmonary functions (FVC, FEV1) post-treatment is similar to previous studies.39 Yoo et al39 employing a mobility protocol for patients with respiratory failure in ICU showed that both ICU stay and hospital stay were shortened.Sprenkle and Michael40 found that early mobilization and upright sitting was favorable for patients admitted to neurological ICUs. They stated that mobilization in neurological ICUs could reduce length of stay, hospital acquired infections, and ventilator associated pneumonia. Early mobility for patients with respiratory failure is not only feasible and safe but also prevents or treats the neuromuscular complications of critical illness.38
The results of the study confirmed the effectiveness of the early mobility techniques for the improvement of quality of life as assessed by increase in the median BI score and modified Rankin scale score. This finding is in agreement with previous findings of improved quality of life in patients receiving mobilization program.41 Moreover, early mobilization program for the stroke patients in ICU results in better functional outcomes at hospital discharge.41 The role of nurses in stroke care will increase and it is important that nurses deliver evidence-based stroke care in order to optimise patient outcomes.42
This study did not report any adverse side effects from the early mobilizing protocol for stroke patients in the ICU. In line with other studies, researchers clearly noted that patients receiving mechanical ventilation and supportive therapy could be mobilized very early, within 24 hours of ICU admission. Limitations of the study include the small number of participants, a single group pretest-posttest design, which precludes further large-scale generalization of results; the relatively short intervention time. Large number of study participants comprised of lacunar infarcts which has been shown to have better functional prognosis.43 Therefore, future studies should explore effect of early mobilization on different sub-types of stroke. Furthermore, the protocol to assess outcome measures only once did not allow for determination of time effect. Future studies with randomized trials involving larger samples with defined dose, dosage of modalilities or early mobilization are needed. Long follow-up after trial cessation is needed to examine the durability of protocol effects. Future studies are needed to examine these questions. Replication of this study through comparison with the control group to verify the effect of the protocol is recommended.
In conclusions, this study demonstrates that an early mobility protocol is safe and effective for stroke patients and may be recommended using the current program as a standard protocol in neurogenic ICUs.
Statistics
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003.
Available from www.icmje.org
Describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to verify the reported results. When possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). Avoid relying solely on statistical hypothesis testing, such as the use of P values, which fails to convey important information about effect size. References for the design of the study and statistical methods should be to standard works when possible (with pages stated). Define statistical terms, abbreviations, and most symbols. Specify the computer software used.
Acknowledgements
We would like to thank the patients who participated in this research.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company. This work was funded by Sheikh Abdullah Al-Tuwaijri Chair for stroke, Majmaah University, Al Majma’ah, Kingdom of Saudi Arabia
- Received September 22, 2018.
- Accepted January 10, 2019.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.