Abstract
Objectives: To investigate the treatment of iatrogenic cerebrospinal fluid (CSF) leak that develops after degenerative lumbar spinal surgery with a subfascial drainage and clipping (SDC) technique.
Methods: This study retrospectively reviewed the medical records of 46 patients who developed iatrogenic CSF leak after surgery for lumbar degenerative spine disease from 2007 to 2019. Twenty-five patients were treated with the SDC procedure (SDC group), whereas 21 were not (control group). Outcomes were compared between the two groups.
Results: CSF leakage ceased within 6–9 days (average 7.4±1) after the procedure in the SDC group. In the control group, CSF leakage was controlled with conservative measures in 14 patients, and in 7 patients, lumbar external drainage was performed. Among these 7, the CSF leak was controlled by lumbar external drainage in 3, and 4 required reoperation to repair the dural defect. No infection occurred in either group. Length of hospital stay was also shorter in SDC group (8.4±1 vs 10.0±1.3 days, p < 0.001).
Conclusion: The SDC technique is effective for the treatment of iatrogenic CSF leak that develops after degenerative lumbar spinal surgery.
Iatrogenic cerebrospinal fluid (CSF) leaks are one of the most common surgical complications of spinal surgery. Incidental dural injury is common during spinal surgery, epidural injection, and myelography. Previous studies have reported incidence rates ranging between 1% and 17% for incidental durotomy during surgery,1 and Gerardi et al2 reported a 6.8% incidence of intraoperatively undiagnosed CSF leak. As many patients with this condition are asymptomatic, it is difficult to predict CSF leaks that are not diagnosed at the time of surgery. Patients with symptomatic CSF leaks may suffer intracranial hypotension-related vertigo, posture-related headache, photophobia, double vision, neck stiffness and dizziness.
Patients who are not diagnosed at the time of surgery or undergo inadequate dural repair may develop a postoperative dural leakage or pseudomeningocele.3In 1983, Teplick and Haskin4 reported a pseudomeningocele incidence of 1.6% detected by computerised tomography imaging among 750 patients who underwent lumbar spinal surgery and remained free of dural leak. When they occur, cutaneous leakage usually develop between the first and seventh days after surgery.
In spinal CSF leaks, oversuturing the incision and application of a pressure dressing may suffice in most cases. When these measures fail, bed rest in the semi-Fowler’s position is recommended. The main target of bed rest is to reduce the CSF hydrostatic pressure in the lumbar region. In 2 previous studies, Wang et al2 systematically prescribed short-term (2.9 days) bed rest, and Camisa et al2⇓⇓ prescribed bed rest for 3–5 days. In addition, acetazolamide,5 repair with blood patch, and closed lumbar subarachnoid CSF drainage can be used. Kitchel et al reported that closed subarachnoid CSF drainage is an effective technique for treatment of postoperative CSF leaks and can prevent a repeat surgical intervention.6 Despite this, the outcomes are not always favourable. When these measures fail, a second surgery for primary dural repair can be performed.
Cain et al7 examined the biology of a dural CSF leak repair in a canine model. They reported that fibroblastic bridging started on the 6th day and dural defects were healed on the 10th day.
We did not encounter any other study in the literature that described the subfascial drainage and clipping (SDC) technique that we perform to treat CSF leaks after degenerative lumbar spinal surgery and report our experience herein.
Methods
We retrospectively reviewed the medical records of 1423 patients who underwent surgery for lumbar degenerative spine disease at Ankara Oncology Training and Research Hospital, Ankara Private Liv Hospital, and Ankara Private MedicalPark Hospital between 2007 and 2019 and identified 46 patients who developed iatrogenic CSF leakage (3.2%). Ethical approval was obtained from the Ankara Oncology Training and Research Hospital Local Ethics Committee for Clinical Research. Informed consent was obtained from each patient. The study was in accordance with the principles of Helsinki Declaration.
Twenty-five patients underwent SDC (treated between 2013-2019), whereas 21 did not (treated 2007-2012). The study excluded patients who underwent intentional durotomy during surgery due to intradural pathology. We included patients with intraoperative dural tears who developed a CSF leak despite dural tear repair and those who acquired intraoperative dural defects of which their surgeons were unaware who developed a postoperative CSF leak. All patients received antibiotic prophylaxis prior to surgery and had a negative pressure #14 Hemovac® drain (Zimmer, Surgical Inc., Dover, OH, USA) placed into the subfascial compartment. The fascia, subcutaneous tissue and skin layers were firmly sutured (watertight closure). On the second postoperative day, fluid discharge into the drain was assessed; if the fluid volume was larger than expected, its colour was lighter, and it showed the halo sign when dropped on a sponge, a CSF leak was diagnosed.
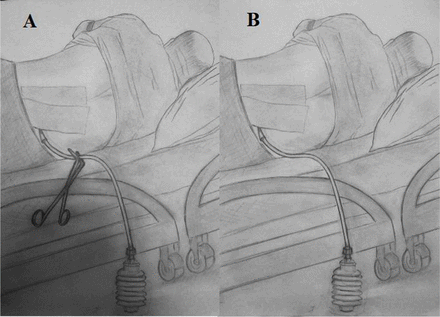
Among patients undergoing the SDC procedure, the drains were clipped on day 2 in order to equalise the intradural and extradural pressures. The drain was clipped and kept closed for 4 h (Figure 1A), then opened for 10 min to allow the fluid drainage (Figure 1B) without any negative pressure, and then re-clipped. In all patients regardless of the drainage technique, acetazolamide 250 mg three times daily was started simultaneously, bed rest in the semi-Fowler’s position was ordered, and pressure dressings were applied. In order to prevent patients from straining, they were prescribed laxatives. This treatment protocol was continued until the fluid drainage decreased to 20–30 mL per day. Then, the drain was removed and the drain site sutured. The patients were monitored for development of a subcutaneous fluid collection for 2 days after drain removal. In the control group, the subfascial drain was removed on the second day after surgery, and the drain site was sutured. As in the SDC group, acetazolamide, bed rest and pressure dressings were similarly utilised. The patients in the control group were then monitored for 7–10 days. All patients in both groups received broad-spectrum antibiotics until drain removal.
Illustration of the subfascial drainage and clipping (SDC) technique. A) subfascial drain is clipped (4 h) B) clamp is removed and spontaneous drainage is allowed (10 min).
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA). Data are presented as mean±standard deviation for parametric continuous variables, percentage for categoric variables. Two group comparisons were carried out with student t-test for parametric, chi-square and Fisher’s exact test for categoric variables. P-values less than 0.05 was considered significant.
Results
The mean patient age was 60.5 years (26–80). Twenty-four patients were male, and 22 were female. Fifteen patients (32.6%) had a history of previous spinal surgery. Comparative analysis of 2 groups are presented in Table 1. In the 25 patients in the SDC group, CSF leakage ceased within 6–9 days after the procedure. Among the 21 patients in the control group, 14 experienced cessation of CSF leakage within 10 days. The 7 patients with persistent CSF leakage at this point underwent closed lumbar subarachnoid external drainage, which succesfully treated the leak in 3.
Comparative analysis of 2 study groups.
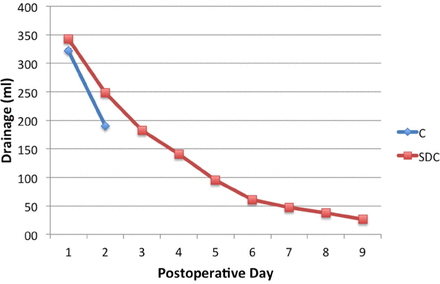
The other 4 patients required revision surgery for dural repair due to worsening subcutaneous swelling and/or persistent CSF leakage occurring from the wound site. None of the patients in either group developed infection. Average daily drainage output of two groups are presented in Figure 2. For the first 2 days, daily drained volume were higher in SDC than control group (Table1). Length of hospital stay was also shorter in SDC group (8.4±1 vs 10.0±1.3 days, p < 0.001).
Daily drainage output in 2 groups. C - Control group, SDC - Subfascial drainage and clipping group
Discussion
The incidence of incidental durotomy during spinal surgery has been previously reported to range between 1% and 17%.1 Our study revealed an incidence of 3.2%. Dural leaks are generally associated with complex spinal surgery and revision surgery. The incidence of dural tears increases with patient age and interventions aimed at spinal stenosis. Intraoperative dural tears resulting in CSF leakage should be primarily repaired, and suturing is usually sufficient. Sometimes a CSF leak is not diagnosed during surgery, or it is inadequately repaired intraoperatively. Even experienced surgeons may not notice pinhole-sized durotomy lesions. The incidence of unrecognised intraoperative durotomy has been reported to be 6.8%. The CSF leaks can be prevented by reducing CSF pressure and/or increasing epidural space pressure, making CSF accumulation difficult as the fibroblastic healing reaction begins. Reducing CSF pressure requires bed rest in the semi-Fowler’s position, closed external lumbar subarachnoid drainage, and acetazolamide administration, which reduces the rate of CSF production.
The reported success rate of external subarachnoid drainage ranges between 85% and 94%.8 However, the complication rate can be as high as 44%, with the most severe complications being excess drainage, pneumocephaly and meningitis. Seventeen percent of patients complain of headache due to excess drainage or nerve root irritation due to catheter placement. In our series, CSF leakage was stopped after lumbar drainage in 3 of the 7 patients who underwent subarachnoid drainage in the control group, whereas revision surgery was required in the other 4. In the SDC group, on the other hand, lumbar drainage was not needed.
Firm fascial closure is used to treat CSF leaks by increasing epidural pressure, delaying CSF flow and facilitating adherence of the dural flaps at the durotomy site. Various views have been reported regarding drain usage. Subfascial drain usage and excessive CSF drainage usually eliminates dead space.9 Cammisa et al2 used drains on a case-by-case basis, depending on the dural leak size, how effectively a leak was repaired, and tissue quality. Eismont et al2 recommended subfascial drain use as it prevented durocutaneous leakage development. Hughes et al5 repaired CSF leaks by use of a negative-pressure subfascial drain that was left in place for 10–17 days. In our study, the drain was in place for only 6–9 days in the SDC group. The advantages of this technique is multifold. The combination of subfascial drainage with clipping technique i) balances the intradural and extradural pressure gradients, ii) prevents formation of dead space and thus allows granulation tissue to form more effectively iii) prevents continuous CSF drainage, and minimizes the risk of headache, nausea and other signs of intracranial hypotension. On the other hand, it is obvious that it may increase the risk of infection, at least theoretically. Nevertheless, we have not encountered any infection in our series of 25 patients treated with SDC technique, therefore it could be regarded as a safe management technique for CSF fistulas following lumbar spine surgeries.
In addition, patients who underwent SDC did not require revision surgery for CSF leakage nor develop pseudomeningocele. Among the patients in the control group who did not receive SDC, however, 4 required revision surgery: one for a pseudomeningocele and 3 for persistent CSF leakage. These results demonstrate that the dural defect is closed more rapidly, and more favourable outcomes can be achieved due to abovementioned benefits in the SDC group.
Although the current study clearly shows the preliminary benefit of SDC technique in the ma-nagement of postoperative CSF leaks after lumbar spine surgery, it has several limitations as well. First, it is a retrospective study with relatively small number of patients. Second, we have not randomized the patients although the baseline characteristics were similar in 2 groups. Nevertheless, we believe that these preliminary results are encouraging, and therefore, a larger prospective randomized trial investigating the potential role of SDC technique versus conventional drainage is war-ranted.
In conclusion, CSF leak repair with SDC is an effective treatment modality that shortens hospital stay and precludes the need for a second surgery.
Illustrations, Figures, Photographs
All figures or photographs should be submitted in a high resolution (minimum 300 DPI) electronic version saved in jpeg or tiff format. Original hard copies of all figures may be requested when necessary. Photographs will be accepted at the discretion of the Editorial Board. All lettering, arrows, or other artwork must be done by an artist or draftsman. If arrows are used please ensure they appear in a different color to the background color, preferably black with a white border, or white with a black border. If arrows distinguish different items on the figure then different arrow styles should be used ie. long, short, wide, narrow. Written informed consent for publication must accompany any photograph in which the subject can be identified. Written copyright permission, from the publishers, must accompany any illustration that has been previously published.
Acknowledgements
The authors would like to thank Enago (www.enago.com) for the English language review.The authors would like to thank Elnur Safarov and Nuray Abasova for the wonderful illustrations.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received July 11, 2019.
- Accepted September 26, 2019.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.