Abstract
Objectives: To identify the dysregulated functional miRNAs, miRNA-16, miRNA-143, and miRNA-200 as potential biomarkers of cerebral aneurysms(CAs) to aid in diagnosis and prognosis.
Methods: This is a prospective case-control study conducted among patients with CA. All patients’ computed tomography angiography (CTA) and interventional angiogram were assessed and studied. The miRNAs were isolated and quantified from peripheral blood samples and the expression profiling was done using TaqMan chemistry on Real-Time PCR.
Results: A total of 37 samples were included. Three cases had double aneurysms and 10 cases presented with aneurysmal rupture. miRNA-16, miRNA-143, and miRNA-200 were upregulated with an absolute fold-change of >8 in the CA group in comparison to controls (p<0.05). miRNA-200 showed double expression in patients with single aneurysm. A statically significant increase was observed in the expression of miRNA-143 in patients who had an aneurysmal rupture with p<0.05. Diabetic patients showed an obvious increase in miRNA-200 (13.03 folds) and miRNA-16 (26.82 folds) expression. Also, there was a notable elevation in miRNA-16, miRNA-143, and miRNA-200 in patients who had hypertension in comparison to those who did not.
Conclusion: miRNA-16, miRNA-143, and miRNA-200 showed statically significant higher expression among cases with cerebral aneurysms in comparison to controls. Thus, these preliminary results of miRNAs biomarkers are promising future tool to be used for aneurysmal screening.
Intracranial aneurysm (IA) is a cerebrovascular disease characterized by abnormal dilation of blood vessels in the brain, which can result in a subarachnoid hemorrhage (SAH) if the aneurysm ruptures. The majority of Cerebral aneurysms (CAs) are asymptomatic, and they are usually discovered incidentally during a routine workup for other complaints. Yet, they raise a significant health concern mainly because of the risk of SAH secondary to their rupture.1
It is estimated that the worldwide prevalence of CAs is around 3.2%, and they are most commonly among ages of 35 and 60 years, with a mean age of 50 years and Female to male ratio of 3:2.1,2 Patients with SAH have a mortality rate ranging between 40-50%, and those who suffer a disability and become dependent range between 20-60%.3 Globally, the incidence of SAH is reported to be 6-10%; however, in Saudi Arabia, it is observed to be only 1-2%.4 Saudis frequently experience IAs that are morphologicaly more complex and typically affect younger individuals, raising the potential of an unidentified underlying vasculopathy cause.5,6
The main diagnostic modalities for CAs include computerized tomography angiogram (CTA), magnetic resonance angiogram (MRA), and digital subtraction angiography (DSA).7 However, due to the lack of precise predictive models for clinically significant growth and rupture, it is still challenging to come up with a comprehensive, cost-effective recommendation for screening and follow-up of patients with unruptured aneurysms. Only a few research have concentrated on linkage analysis and a few number of genes have been identified with differential expression in aneurysm tissue.8,9
Micro-RNAs (miRNAs) are short, 22 nucleotide-long molecules that function as targets for translational repression or cleavage of other miRNA. Considering their inert properties, some studies have shown a potential benefit in employing miRNA expression profiles as biomarkers in the identification and prediction of various aneurysm types. Studies have determined that the miRNA signature in blood is similar in both sexes as well as in individuals of different ages.10,11
Several miRNAs have been shown to play potential roles as biological markers for CAs. However, the absence of established procedures in investigations of circulating miRNAs as biomarkers may give rise to bias in the interpretation of data.12 Some studies have indicated that certain miRNAs play potential roles in the pathogenesis of CAs and their subsequent complications. For example, miRNA-16 has been linked to the formation and development of CAs. Because miRNA-16 is expressed by vascular endothelial cells associated with angiogenesis, it could be used as a potential marker for assessing CA risk.13
Recently, Meeuwsenet al14 has shown that miRNA-200a-3p was increased only in SAH patients, which suggests that miRNA-200a-3p influences the risk of aneurysmal rupture and could be used for aneurysmal rupture risk assessment. Furthermore, miRNA-143 was significantly downregulated in patients with CAs.14 However, Bekeliset al15 found that cerebral aneurysmal tissue showed other miRNA types of expression. In fact, this downregulation is believed to play a central role in contributing to vascular smooth muscle cell phenotypic modulation, which is essential for the formation of intracranial aneurysms.15 Moreover, The International Study of Unruptured Intracranial Aneurysms Investigators (ISUIA) has recommended early screening and prophylactic treatment for patients with no history of CA rupture to significantly improve prognosis.16
Therefore, the present study aims to evaluate the expression of miRNA-16, miRNA-200a-3p, and miRNA-143 in patients who suffer from CAs as potential biomarkers to be used in early detection, monitoring the progression of an aneurysm, and determining a promising prognosis.
Methods
Study design and population
This prospective case‒control study evaluated the miRNA expression profiles of miRNA-16, miRNA-200, and miRNA-143 in patients with CAs. All patients were admitted to King Fahd Hospital of the University, Al Khobar, over a duration of 18 months between August 2018 and February 2020. The diagnosis of CA was confirmed using digital subtraction angiography. The clinical history of all patients was collected on a predesigned patient data sheet. The control group comprised 20 healthy, age- and sex-matched controls with no history or family history of CA. All patients’ reports from CTA and DSA procedures that were part of their case management were reviewed.
Inclusion and exclusion criteria
The inclusion criteria of the present study included the following: Age >18 years, and confirmed diagnosis of CA. However, patients with a recent head injury, the presence of any other type of cerebral vascular abnormalities, extra-axial or intra-axial brain tumors, neurological diseases such as multiple sclerosis, or Parkinsonism, or previous history of stroke were excluded.
Ethical approval
This current study was approved by the Ethical Committee of Imam Abdulrahman bin Faisal University and followed the 1964 Helsinki Declaration and its later amendments. Every participant or the next of kin for patients who were incapacitated signed an informed written consent following the Institutional Review Board (IRB-UGS-2018-01-037, approval date 31/1/2018) rules and regulations.
MiRNA expression profiling
Three milliliters of blood were collected from each subject in EDTA-coated tubes for miRNA expression profiling. In ruptured aneurysms, blood samples were obtained at the acute stage, and in the rest of the cases, samples were collected after management.
miRNAs were isolated from peripheral blood samples using the mirVana™ miRNA Isolation Kit (Ambion, Austin, USA), which allowed total RNA containing the miRNA population to be isolated. The miRNA quality and quantity were evaluated using a Nanodrop ND-8000 spectrophotometer (Thermo Scientific, Wilmington, USA) and Qubit™ microRNA Assay Kit (Invitrogen, Carlsbad, CA), respectively. All the samples were diluted to a final concentration of 20 ng/µl. The samples were used immediately or stored at −80 °C for future use.
The TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used for the preparation of cDNA. Reverse transcription reactions were performed on a C1000 Touch™ Thermal Cycler (Bio-Rad) with the following conditions: 16 °C for 30 min, 42 °C for 30 min, 85 °C for 5 min, and 4 °C on hold. TaqMan assays using a fluorescence tagged TaqMan microRNA probe for 3 miRNAs were performed on a QuantStudio 3 Real-Time polymerase chain reaction (PCR) system. Real-time PCR cycling conditions consisted of 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 s and 60 °C for 1 min. The cycle threshold (CT) defined by proprietary software of the Real-time PCR was exported to an Excel document for further analysis. The cycle threshold Ct-values of the 3 miRNAs were normalized by subtracting the average Ct-value of the housekeeping miRNA, and the Ct method of relative quantification was used to calculate the fold change expression of miRNAs.17
Statical analysis
The clinical data along with studied miRNA expression (qualitative) results were analyzed using SPSS version 23 (IBM Corp.). Distribution of proportions across category variables were analyzed using a Fisher’s exact test, and for continuous variables an unpaired Student’s t-test was used. The relationships between the variables were determined using a Spearman’s Rank Correlation test. Statistical significance was considered for p<0.05.
Results
Demographic characterization of the patients
Initially, a total of 36 CA cases and 40 controls were recruited; however, at the start of 2020, all universities closed as a result of the COVID-19 pandemic. Once the university reopened, work resumed, but all blood specimens collected during that time were excluded due to the deterioration of the samples. Therefore, only blood specimens that were analyzed before the lockdown were included. As a result, only 17 cases (10 females and 7 males) and 20 controls (10 males and 10 females) were included in the study. The mean age of the patients was 45.1 years, while for the controls, it was 45.5 years. Among the 17 CA patients, 17.6% (3 cases) had multiple aneurysms, while the remaining 82.4% had single aneurysms. Aneurysmal rupture was seen in 58.8% (10 cases), and among these 10 cases who presented with aneurysmal rupture, 50% of them developed vasospasm. A total of 12 CA patients underwent aneurysmal coiling, while the remaining 5 underwent clipping procedures. Some comorbid and causative conditions, such as diabetes mellitus (n=4), hypertension (n=9), and smoking (n=5), were also observed in the patient group. All baseline data and the clinical and radiological findings are summarized in Table 1.
- Clinical data and expression profiling of CA cohort.
miRNA expression
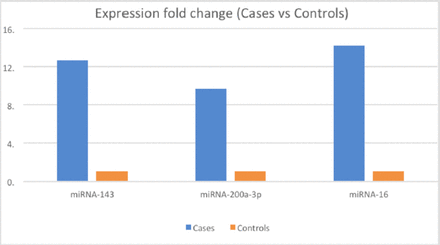
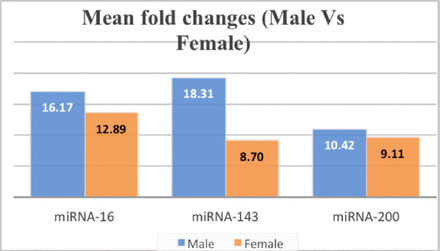
miRNA-16, miRNA-143, and miRNA-200 were upregulated significantly with an absolute fold-change of >8 in the CA group (Figure 1) compared to the normal group (p<0.05). Sex-based analysis showed that all miRNAs levels were higher among male patients in comparison to female patients, especially miRNA-143, which showed almost double the expression in males in comparison to females (Figure 2).
- This graph shows the miRNAs expressional fold changes between healthy controls and CA cases, the mean expression of the controls was set as 1 in which we considered it to be the reference point to measure the expressional fold changes of the cases in regard to this reference point.
- MiRNA expression of CA cases based on gender, showing almost the doubling of miRNA-143 in males in comparison to females.
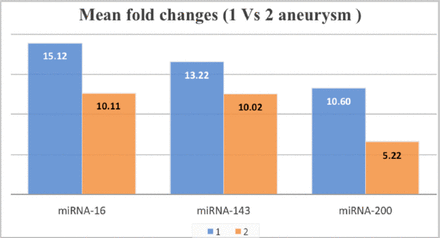
MiRNA expression levels were higher for all 3 miRNAs among cases with a single aneurysm compared to those with two aneurysms. The highest fold change was observed in miRNA-200 and showed almost double the expression in the single aneurysm group compared to the two aneurysms group (Figure 3).
- miRNA expression based on the multiplicity of aneurysms, showing higher expression among cases who had a single aneurysm in comparison to those who had two aneurysms.
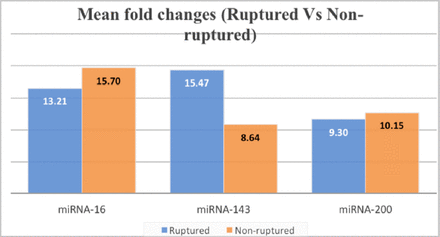
In addition, there was an obvious increase in miRNA-143 expression levels in patients who had an aneurysmal rupture, almost twofold higher than in those who did not suffer a rupture (p<0.05). Conversely, miRNA-200 and miRNA-16 expression were slightly higher in patients who did not have aneurysmal rupture (Figure 4).
- miRNA expression based on the presence aneurysmal rupture, miRNA-143 was highly expressed among cases who had aneurysmal rupture.
MiRNA-143 and miRNA16 expression levels were almost 3-fold higher among patients who did not have a previous history of stroke. Patients with HTN had almost doubled expression levels for all 3 miRNAs with an obvious increase in miRNA-16 (Table 2). Patients with DM had an increase in the expression of all miRNAs but more dramatically in miRNA-200 and miRNA-16. Additionally, there was a clear elevation in all miRNAs in patients who smoked. Reduced expression of the 3 miRNAs was noted in patients who developed vasospasm. However, the comparison of miRNA expression among these sub-groups failed to exhibit statistical significance (p<0.05) due to the small sample size.
- Mean fold change of miRNA expression in comorbid conditions
Discussion
The incidence of ruptured aneurysms among our sample was found to be 58.8%, which is higher compared to earlier studies that reported an incidence of 10 per 100,000 in the normal population.18 The higher incidence rate of rupture in our study could be attributed to the size, location, and shape of the aneurysms and older age. However, we must consider factors other than morphological aspects of the aneurysms as risk factors, as there were personal and clinical factors that have also been found to be significantly associated with a higher risk of aneurysm ruptures, such as cigarette smoking, alcohol consumption, and hypertension.19 In our sample, 29.4% of the patients were active smokers, and 52.9% were hypertensive, which might explain the higher incidence of rupture in our group. The male-to-female ratio among our patients was 7:10, with a female predominance. This finding is in line with previous studies in which a male-to-female ratio of 31:53 was reported in an autopsy study with a higher prevalence in women.20 Another meta-analysis showed that the prevalence of unruptured intracranial aneurysms was double among women compared to men.21
Around 70% of human miRNAs are currently known to occur in the brain. In fact, it has been suggested that they control the expression of roughly half of human genes, and they have an impact on almost all cellular pathways. This in turn makes miRNAs an excellent target for research that concerns with central nervous system diseases.22 In vitro experimental studies have demonstrated that miRNA is crucial for endothelial cell gene expression and function. It is believed that more than 1,000 distinct miRNAs reside in individuals, most of which are expressed in a tissue- or cell-limited manner. miRNA can be identified in serum and plasma in a surprisingly precise way. Measurement of circulating miRNAs can shed some light on clinical outcomes of interest.23
Several studies demonstrated related changes in expression of circulating miRNAs in patients with IA. Feng et al24 found that the plasma levels of miRNA-143/145 were downregulated in patients with IA in comparison to healthy controls. Thus, it has been suggested that low miRNA-143/145 expression was associated with formation and progression of IA. Also, Yang et al25 revealed that patients in the non-IA group showed a rise in serum relative expression of miRNA-155 compared to the IA group with rupture. Therefore, IA rupture can be predicted using a change in serum miRNA-155 expression. Additionally, another study showed that patients with aneurysmal SAH had significantly altered level of plasma expression of miRNA-15a-5p, miRNA-34a-5p, miRNA 374a-5p, miRNA-146a-5p, miRNA-376c-3p, miRNA-18b-5p, miRNA-24-3p, and miRNA-27b-3p.26
In our study, miRNA-16, miRNA-143, and miRNA-200 were upregulated by more than 8-fold in the CA group compared to the controls. Previous studies have identified miRNA expression as an independent predictor for the presence of intracranial aneurysms without understanding the origin of its elevation and suggested its use as a novel biological marker in evaluating the probability of occurrence of CAs in high-risk individuals.13 miRNA-143 was also found to be the most significantly downregulated miRNA in patients.15 Our cohort showed a 12-fold upregulation of miRNA-143, which is at odds with some of the previous studies. miRNA-143 levels were higher among patients with ruptured aneurysms, suggesting that miRNA-143 influences the risk of aneurysmal rupture. One explanation is its role in vascular smooth muscle cell phenotypic modulation, which is crucial in CA formation.27 miRNA-200a-3p was previously reported to be involved in the development and rupture of intracranial aneurysms when compared to controls.13,15
Furthermore, our study indicated that all three miRNA expression levels were higher among patients who had a single aneurysm than among patients with multiple aneurysms. This also suggests that there might be another cascade with different miRNAs involved that is responsible for the formation of multiple aneurysms in a single patient.
In the current study, significantly higher expression levels of miRNAs from peripheral blood specimens were observed in patients with CA than in healthy controls, indicating the involvement of the overexpression of these miRNAs in this disorder. Therefore, circulating microRNAs might help guide the diagnosis of different types of aneurysms. However, the main finding in the present study is that compared with healthy controls, plasma miRNAs are significantly upregulated in patients with cerebral aneurysms, regardless of the type of aneurysm (ruptured/unruptured or single/multiple). We tried to minimize as much as possible the biases affecting the validity of our findings. We used a prospective case‒control design, where all cases were age- and sex-matched to controls over a broad period of 18 months, which reduced recall biases and those related to individual differences. However, the limited sample size and single-center recruitment may limit the generalizability of the results.
Accordingly, further research could be conducted in several areas to advance our understanding of the role of miRNAs in cerebral aneurysms and to improve the diagnosis and treatment of this disorder. First, a larger-scale multicentric study could be conducted to validate the findings of this study and to assess the generalizability of the results. Second, longitudinal studies could be conducted to investigate the temporal changes in miRNA expression levels in patients with CAs and to determine whether these changes are associated with disease progression-ruptured vs unruptured CAs-, treatment response, or prognosis. Third, functional studies could be conducted to investigate the mechanisms underlying the overexpression of miRNAs in cerebral aneurysms and to identify potential therapeutic targets for this disorder. Lastly, studies could investigate the diagnostic and prognostic value of miRNAs in different types of cerebral aneurysms and to compare their performance with other biomarkers or imaging modalities.
Conclusion
The results of our preliminary study suggest a promising future for miRNA in CA diagnosis and treatment. Specifically, miRNA-16, miRNA-143, and miRNA-200 show promise as possible biomarkers to discriminate between CA patients and healthy controls by showing statistically significant higher expression among cases with CA in comparison to controls. Further studies are encouraged in this area.
Acknowledgment
We would like to thank Nithya Prabu for her work on data analysis. In addition, we would like to thank American journal Experts for English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received March 26, 2023.
- Accepted August 19, 2023.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.