Abstract
Objectives: To determine the effectiveness and safety of Hemopatch® as a primary dural sealant in preventing CSF leakage following cranial surgery. Cerebrospinal fluid (CSF) leaks occur in cranial operations and are associated with significant patient burden and expense. The use of Hemopatch® as a dural sealant in cranial neurosurgical procedures is described and analyzed in this study.
Methods: Data were retrospectively collected from all patients who underwent a craniotomy for various neurosurgical indications where Hemopatch® was used as the primary dural sealant between June 2017 and June 2022. Infection and CSF leak were the main indicators evaluated after surgery.
Results: A total of 119 consecutive patients met our inclusion criteria. The median was age 41.5 years, and 52.5% were female. The mean follow-up period was 2.3 years (7 months to 6 years). There were 110 (92.44%) supratentorial and 9 (7.56%) infratentorial craniotomies. Postoperative CSF leak was reported in 2 patients (1.68%), one in each cohort. Postoperative infection occurred in one patient (0.84%).
Conclusion: The results suggest that using Hemopatch® as a dural sealant in cranial surgery is effective and safe. After supra-/infratentorial craniotomies, the rate of postoperative adverse events in our sample was within the range of known surgical revision rates. Future randomized clinical studies are required to confirm our encouraging findings.
A cerebrospinal fluid (CSF) leak is one of the most challenging complications that can occur after cranial surgery.1,2 The consequence of this surgical complication includes morbidities, such as infection and pneumocephaly, and increased mortality. The prolonged length of hospital stay and the management of postoperative CSF leak affects the quality of medical care and increases the cost of health care delivery.3,4 The incidence of CSF leakage during craniotomy has been reported to range from 4% to 32%.5 The location and the size of the craniotomy and dural defect can influence the likelihood of CSF leakage after surgery.6 Posterior fossa and skull base craniotomy are associated with an increased likelihood of CSF leak compared with the supratentorial craniotomy. In addition, patient-specific characteristics, such as immunological status, age, and medical history, play a role.7
The dura mater, which is composed of vascular fibroblasts, protects the central nervous system by preventing CSF leaks and invasion by infectious pathogens. A variety of approaches for dural reconstruction after cranial surgery have been reported. Many of these strategies can be used in combination or with other elements to enhance closure.8 Achieving a watertight dural closure and using sealant materials are 2 of these methods. However, there is currently no consensus regarding the benefits and cost-effectiveness of primary watertight dural closure or any other closure approach; this makes it difficult to determine whether these dural reconstruction techniques are necessary.9 Neurosurgeons have long discussed the primary purpose of duraplasty and its many indications. The traditional standard of care for a durotomy is to provide a watertight dural closure; however, this is not always possible due to the fragility of the dura mater and its tendency to retract after a long cranial procedure.
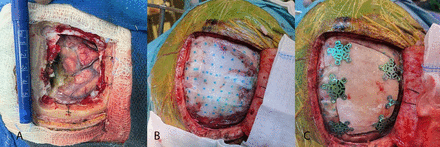
Hemopatch® (Baxter Deutschland GMBH, Germany) is a sheet of polyethylene glycol-coated bovine dermal tissue that possesses hemostatic and sealing properties.10 It has a self-adhering sealing surface that uses a fast protein-reactive monomer to adhere to tissue through covalent amide interactions between polyethylene glycol and tissue proteins and collagen. Figure 1 illustrates the intraoperative use of Hemopatch® as a dural sealant during surgery.
- Demonstration of the intraoperative application of the dural substitute (Hemopatch®). A) Dural defect post-brain tumor resection. B) Dural sealant substitute (Hemopatch®) placement over the dural defect extending over to the craniotomy edges. C) Craniotomy bone flap fixation with microplate and screw technique.
In this study, we evaluated our clinical experience with the Hemopatch® dural sealant in a variety of supratentorial and infratentorial procedures.
Methods
Study design and participants
From June 2017 to June 2022, we retrospectively analyzed patients who underwent craniotomy during which Hemopatch® was used as the primary artificial dura substitute. Institutional ethical approval was obtained (number 2231424). We included a wide range of cranial approaches, with various underlying diseases and emergency procedures. All patients received at least one intravenous antibiotic injection prior to skin incision as a standard. Bone flaps were fixed with screws and plates. Patient records, surgical reports, and postoperative magnetic resonance imaging scans were reviewed. We did not exclude any type of underlying pathology. In every case, the surgeon decided independently, based on his own clinical experience, whether to use a Hemopatch® or another dural substitute to achieve a watertight dural closure. Our primary outcome measure was the incidence of CSF leak, and our secondary outcome was the incidence of surgical site infection. Patients were followed up for at least 6 months after surgery through the clinic.
Data collection
Using electronic medical records, we obtained general patient information, the size of the dural defect (small <0.5 cm, medium 0.5–2 cm, and large >2 cm), and the type of dural closure. Cranial operations were classified as either supratentorial or infratentorial. Another variable we considered was history of previous surgery in the region. In addition, we recorded whether a CSF diversion procedure was performed before or after surgery. From postoperative progress reports and clinic records, CSF leaks were documented as a postoperative complication; they were reported as an observed leak through the incision site. Positive post-surgical cultures relevant to the surgery were reported as surgical site infection. Pseudomeningocele incidence without CSF leak was not analyzed in this retrospective study.
Surgical technique
Hemopatch® was applied according to the manufacturer’s instructions. The self-adhering sealing surface was applied dry to the dural surface, and a moist gauze pad was gently pressed onto it for 30–60 seconds. We applied a technique of extending the sealant over the craniotomy bone edges (Figure 1). No intraoperative specific test, such as the Valsalva maneuver, was utilized to assess for CSF leak after the dural sealant application. Once the bone flap fixed this will ensure the stability of the sealant over the dural defect. In certain cases, the dura was approximated to form a scaffolding for the Hemopatch® to lie on. In all reported cases, Hemopatch® was the primary and exclusive dural substitute without additional sealants.
Statistical analysis
The data analysis was conducted using Microsoft Excel software and statistical percentile comparison. Only patients who received Hemopatch® for primary dural substitute following craniotomy were included in the analyses. Categorical variables are presented as frequencies with percentages applying a descriptive statistical analysis. All statistical analyses were conducted using Stata statistical software (Release 17, StataCorp LLC, 2021, College Station, TX).
Results
Baseline demographics and operative parameters
A total of 119 consecutive craniotomy surgeries (52.5% females, median age 41.5 years) with Hemopatch® as the principle duraplasty element were identified from June 2017 to June 2022, Table 1. One hundred and one (92.44%) craniotomies were supratentorial and nine (7.56%) were infratentorial. The durotomy defect size was between 0.5 and 2 cm in 18 (15.13%) cases and greater than 2 cm in 101 (84.87%) cases. Seven (5.88%) surgeries included CSF diversion (extraventricular drain or ventriculoperitoneal shunting).
- Patient characteristics and outcomes.
Postoperative complications
Postoperative CSF leak was reported in a total of two patients (1.68%); one patient from the infratentorial craniotomy cohort (11.11%) and one from the supratentorial craniotomy cohort (0.91%), Table 1. Neither of the patients needed concomitant CSF diversion. The exact size of the dural defect was not documented. Postoperative infection was reported in one (0.84%) patient from the supratentorial cohort. No allergic reaction to Hemopatch® has been reported. In this study, we did not analyze the incidence of pseudomeningocele. However, no patients underwent any postoperative interventions to treat pseudomeningocele.
Discussion
In reviewing the literature, it is difficult to determine which method of dural closure is superior as there is no consensus on the necessity of the procedure. Depending on the study and the type and site of cranial procedures, the incidence of CSF leak ranged between 1% and 4%.11 Spitaels et al conducted a review and comparative analysis of Tachosil®, DuraSeal®, and other to methods to prevent CSF leak.12 After assessing non-randomized controlled trials, they concluded that fibrin sealants are safe and are effective in preventing CSF leaks. In our study, we were able to demonstrate that Hemopatch® can be used safely as a dural sealant in cranial procedures where a watertight dural suture cannot otherwise be achieved. However, these findings must be interpreted with caution due to their retrospective nature and the lack of comparison to a control group.
One study evaluated the use of Hemopatch® as a dural substitute in patients who had undergone surgery to remove a brain tumor. The researchers found that Hemopatch® was effective in sealing the dural defect, and it did not cause any adverse reactions.13 Furthermore, the use of the Hemopatch® was associated with a shorter hospital stay and a more rapid recovery time compared with traditional methods of dural repair.
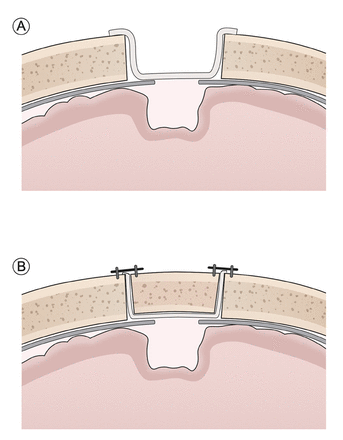
- Illustration of the Hemopatch® application before and after cranial bone flap fixation.
- Summary of the literature evaluating hemopatch® utilization in cranial neurosurgery.
Another study compared the use of Hemopatch® to fibrin glue in a group of patients who had undergone cranial and spinal surgeries.14 The researchers found that Hemopatch® was associated with a lower rate of complications and a faster recovery time compared with fibrin glue. Considering the average rate of surgical site infection in cranial neurosurgical procedures, data from several studies showed that using Hemopatch® does not increase the risk of postoperative infection. The infection rate of 0.84% in our study is considered low and below the reference benchmark. The applied post-craniotomy infection rate benchmark at our hospital is 1.35%, which is the benchmark published by the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).15 One wound infection was observed in our series, which occurred in an elderly gentleman in his 70s with comorbidities, including diabetes and hypertension. He underwent a craniotomy and resection for a left frontoparietal meningioma complicated by a postoperative course of subdural and epidural collection positive for Propionibacterium acnes that was treated with surgical evacuation and a course of antibiotics. Overall, the evidence suggests that Hemopatch® may be a valuable alternative to traditional methods of dural repair in neurosurgery.16,17 It offers several advantages, including ease of use, durability, and a lower risk of complications. Further research is needed to fully understand the potential benefits and risks of the Hemopatch® as a dural substitute; however, the initial evidence is promising.
There are certain limitations of this study that include the retrospective nature of our data collection and the absence of a control group limits the comparison of the outcomes. The same surgical Hemopatch® application method was used by all surgeons; however, all patients were not operated on by the same surgeon, and this may have introduced variability in the delivered surgical technique. Finally, this study was not aimed at determining whether this method of closure is superior to other dura sealant techniques.
Conclusion
Our retrospective clinical observation of the intraoperative application of Hemopatch® demonstrated its efficacy, safety, and practicality as a dural substitute. The rates of CSF leakage and infection were not significantly different between the Hemopatch® dural analogue and reports of primary dural closure presented in the literature. A prospective randomized controlled trial would be required to confirm our promising results supporting Hemopatch® as the primary dural sealant alternative in neurosurgery.
Acknowledgement
We would like to thank the Saudi Epilepsy Society Academy for supporting the production of this manuscript.
- Received November 26, 2023.
- Accepted February 20, 2024.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.