Abstract
This review discusses the mechanisms of action of 4 immune modulating drugs currently used in the treatment of multiple sclerosis (MS), including Alemtuzumab, a humanized monoclonal antibody that functions by targeting CD52, an antigen primarily expressed on T and B lymphocytes and monocytes/macrophages, resulting in their depletion and subsequent repopulation; Dimethyl fumarate that switches cytokine production toward a T helper 2 profile and enhances cytosolic levels of nuclear factor erythroid 2–related factor 2, which has immune regulatory and cytoprotective effects on oligodendrocytes, neurons, and glial cells; Fingolimod functions by blocking the release of activated lymphocytes from lymph nodes by targeting sphingosin-1-phosphate receptors; Natalizumab a humanized monoclonal antibody binds a4b1-integrin resulting in reduced migration of immune cells from blood across the blood-brain barrier into the CNS. This review presents the most up to date information on mechanisms of action, safety, and efficacy of these immune modulators and provides future perspectives for the treatment of MS.
In patients with multiple sclerosis (MS) fatigue is rated one of the most common and disabling symptoms. Its prevalence ranges from 65-97%, and it tends to seriously impair approximately one-third of all MS patients.1 It is assumed that MS is a disease of the immune system primarily characterized by the infiltration of autoreactive immune cells into the CNS. It has been demonstrated that these autoreactive immune cells are the root cause of neuronal loss, gliosis, demyelination, and ultimate cerebral atrophy.2,3 Secondary causes such as sleep problems, medication, and depression have also been suggested to be associated with MS-related fatigue.4,5 Most MS patients experience a relapsing-remitting course, which is characterized by a recurrent series of self-limited inflammatory activity. Involvement of a specific part of the CNS results in bouts of neurological deficits or relapses that manifest clinically.6
Lymphocyte including interleukin (IL)-17–producing T-cells have been observed in active MS lesions in the CNS. In patients with MS, the suppressive function of regulatory T-cells function to suppress autoreactive T-cell proliferation through cytokine production and contact with effector T-cells or antigen-presenting cells is impaired.7,8 Although the precise function of B-cells in MS pathogenesis is unknown, it likely involves antigen presentation, cytokine production, and/or immunoglobulin synthesis.9
Multiple sclerosis is a disease that had no treatments that modified its course until the early 1990s when interferon beta (b) was introduced. Injection and infusion drugs remained the mainstay of MS treatments for almost 2 decades when finally oral therapies were developed.10 The interferons are the first-line injectable drugs used for MS. Injection-site reactions, flu-like symptoms, and liver dysfunction lead to the risk of developing neutralizing antibodies, which limits their effectiveness. Therefore, new orally administered drugs were approved for MS treatment. Dimethyl fumarate (DMF), marketed as Tecfidera®, has now been granted approval for MS treatment by the US Food and Drug Administration. Various oral drugs, which have been approved by regulatory agencies for the treatment of MS, their mechanisms of action, efficacy, and safety are reviewed herein.
Pro- and anti-inflammatory cytokines
Multiple sclerosis is an autoimmune inflammatory disorder of the CNS, in which autoreactive T-lymphocytes recognize CNS-specific proteins resulting in inflammation, demyelination, and axon degeneration.11 The pro- and anti-inflammatory cytokines are up-regulated in most MS patients. The MS patients display increased serum and CSF levels of pro-inflammatory cytokines such as interferon gama IFN-g, tumor necrosis factor-alpha (TNF-a), lymphotoxin-a, IL-2, IL-1b, and anti-inflammatory cytokines such as IL-10, IL-13, and transforming growth factor-beta that have been linked to fatigue.12 The MS-related fatigue may be some form of inflammation-induced sickness behavior resulting from cytokine-induced changes in CNS neurophysiology. The administration of immunomodulatory medication such as interferon-beta (IFN-b) frequently causes short-term effects such as reversible fatigue in MS.13 Glatiramer acetate is used in the treatment of MS, and has anti-inflammatory properties and reduces fatigue in MS patients. Natalizumab treatment reduces circulating plasma levels of TNF-a, IL-6, and IL-10 as well as CSF levels of IL-1b, IL-6, and IL-8, and seems to have a beneficial effect on subjective fatigue in MS patients.14 Aerobic exercise leads to a reduction in fatigue in MS patients by anti-inflammatory actions.15
The T follicular helper (TFH) cells are important for the activation of B-cells in secondary lymphoid tissues, and increased TFH cell and B-cell activation is found in patients with MS.16 A study of CSF from patients treated with fingolimod, found that CD4+ T-cells were the main lymphocyte subtype reduced.17 CCR7+ CD4+ T-cells were reduced in the CSF from patients having a relapse early after the initiation of fingolimod treatment. Interestingly, half the patients exhibited increased circulating Th17 cells and half showed reduced circulating Th17 cells, suggesting variability among patients.18
Alemtuzumab
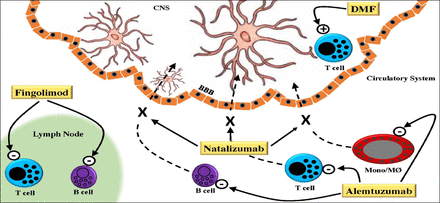
Alemtuzumab is a humanized monoclonal antibody therapy for relapsing-remitting multiple sclerosis (RR-MS). It acts by targeting CD52, an antigen primarily expressed on T and B lymphocytes and resulting in their depletion and subsequent repopulation (Figure 1). Human lymphocytes are also susceptible to complement-dependent cytolysis after Alemtuzumab exposure, at least in vitro.19
Schematic representation of the mechanisms of action of 4 immune modulating drugs currently used in the treatment of multiple sclerosis (MS). In the circulatory system Alemtuzumab targets CD52, primarily expressed on T and B-cells and monocytes (Mono)/macrophages (MØ) resulting in their depletion. Also in the circulatory system, Natalizumab binds to a4b1-integrin on T-cells, B-cells and Mono/MØ resulting in their reduced migration across the blood-brain barrier (BBB) into the central nervous system (CNS). Fingolimod blocks the release of activated lymphocytes from lymph nodes by targeting sphingosin-1-phosphate receptors. In the CNS, dimethyl fumarate (DMF) switches cytokine production of T-cells that have migrated into the CNS towards a Th2 profile and enhances NRF2, which has immune regulatory and cytoprotective effects on oligodendrocytes, neurons, and glial cells.
The therapeutic effect of Alemtuzumab is likely not solely a consequence of lymphocyte depletion, but also of repopulation features. Animal studies have shown that lymphocyte numbers in primary and secondary lymphoid organs are maintained.20 Innate immune cells, some T-cell subsets (tissue-resident effector memory T- cells), plasma cells, and serum immunoglobulin levels are unaffected by Alemtuzumab.20 The relative proportions of regulatory T-cells and memory-phenotype T-cells are increased, and the proportion of naive T-cells is decreased during repopulation.20 The proportion of B -cells with a mature naive phenotype was reduced after treatment, whereas the immature cell fraction increased. Also, there were decreases in the proinflammatory cytokines IFN-g, IL-12, and IL-27.20
Despite effective suppression of inflammation, more than half the patients had a sustained increase in disability and/or had further brain volume loss during an 18-month follow-up period.21 Although axonal degeneration in patients with secondary progression occurs largely in the absence of inflammation, the focus shifted to treating patients earlier in their disease course. Patients with relapsing-remitting disease were targeted in the phase II and III studies. For long-term efficacy, the patients completing the phase II and III trials were eligible to continue in an extension study [ClinicalTrials.gov identifier: NCT 00930553] in which they could receive as-needed Alemtuzumab retreatment.22 In a 5-year follow-up of the CAMMS223 extension, the risk of SAD from baseline to year 5 was reduced by 69% (p=0.0005) in the Alemtuzumab 12-mg group relative to the SC IFNB-1a group.23 From year 3 to year 5, there was a 56% relative reduction in relapse rate, but this failed to reach significance (p=0.09).23
In phase II and III studies, adverse events (AE) were reported in 96−100% of patients treated with Alemtuzumab 12 mg.24 During the core studies, 2 deaths occurred in CAMMS223 (cardiovascular disease and idiopathic thrombocytopenic purpura (ITP)), one in CARE-MS I (automobile accident), and 2 in CARE-MS II (automobile accident and aspiration pneumonia following brainstem relapse). Infusion-associated reactions (IAR) were the most common AE in all 3 deaths and those associated with Alemtuzumab are thought to be mainly attributable to cytokine release syndrome due to target cell lysis and recruitment of pro-inflammatory cells.24 The most common IARs and their incidence with Alemtuzumab-12 mg included headache, pyrexia, nausea, pruritus, insomnia, fatigue, chest discomfort, and dyspnea (Table 1). Serious infections were rare, but the common infections were those of the respiratory tract and urinary tract (Table 1). Herpetic infections, including mucocutaneous herpes simplex and herpes zoster, were increased with Alemtuzumab in the CARE-MS studies, but declined after the introduction of acyclovir prophylaxis as a study protocol amendment.24 A case report of thyroid carcinoma after Alemtuzumab in a patient with otherwise normal thyroid function has also been published.25 Autoimmune AEs represent the most important risk associated with Alemtuzumab treatment due to lymphocyte repopulation.26-28
Comparisons of the effects of various medications on multiple sclerosis patients.
Dimethyl fumarate
Dimethyl fumarate has a multitude of biological effects including anti-inflammatory properties linked to its ability to promote a Th2 immune response (Figure 1). Peripheral mononuclear blood cells enhance the production of IL-4 and IL-5, cytokines of the Th2 phenotype.29-31 In vivo and in vitro experiments have further clarified the impact of DMF on type II dendritic cells on exposure to DMF.32 T-lymphocytes seem to be just a portion of the modifications induced by DMF, which influences several other cells, including macrophages, microglia, astrocytes, and neurons.33
Reports from 2 large phase III studies of DMF treatment revealed that the most common side effects include gastrointestinal symptoms and flushing.34 Rare side effects of DMF treatment include lymphopenia, leucopenia, ketones in urine and a decrease in vitamin D and WBC (Table 1). Over 2 years, the Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting Multiple Sclerosis (DEFINE) study35 was set to assess as the primary aim the proportion of patients who developed clinical relapses after random assignment to either 240 mg of DMF twice a day (BID), or 240 mg 3 times a day (TID), or placebo. The common denominator to those cases was the high degrees of lymphopenia; this correlates with an incompetent immune system, the premise for opportunistic infections to occur.35 With decades of experience with obvious benefits and no significant ill effects, DMF has aspired to the role of an immune therapy with broader applications.36
Natalizumab
Natalizumab (NTZ, Tysabri®), a humanized monoclonal antibody targeting the alpha chain of the αá4βâ1 adhesion molecule approved for the treatment of RR-MS reduced the relapse rate by 68%, and the risk of disability progression by 42%.37 Natalizumab blocks the binding of VLA-4 to vascular cell adhesion molecule 1 (VCAM-1), resulting in reduced migration of immune cells from blood across the blood-brain barrier into the CNS (Figure 1). Natalizumab’s differential effects on lymphocyte subsets might contribute to progressive multifocal leukoencephalopathy (PML) susceptibility. Natalizumab exerts some effects on B-cells by mobilizing CD34C progenitor cells out of bone marrow into peripheral blood and elevates persistently circulating CD19C B-cells,38 and it modifies B subpopulations with a decrease in naïve B-cells and an increase in memory B-cells.39 Anti-JCV antibody levels increase in NTZ-treated patients.40 The proportion of CD4C T-cells expressing L-selectin was lower in long-term NTZ-treated patients than in untreated MS patients and an unusually low percentage of CD4C T-cells expressing CD62L was associated with a higher risk of developing PML.41 A study of B-cells in NTZ-treated patients modulated the expression of a particular set of deregulated microRNAs (miRNAs) found in untreated MS patients.42 In a separate study, analyses of blood miRNA expression patterns during the first 6months of treatment revealed a decrease in let-7c, miR-125a-5p, and an increase in miR-642 expression, suggesting these miRNAs as possible biomarkers for MS.43 The miRNA known as miR-125a-5p plays role in the leukocyte migration process and in the regulation of brain endothelial integrity; miR-320, miR-320b, and miR-629, are related to PML: miR-320 and miR-320b showed higher expression, and miR-629 lower expression in PML patients compared with non-PML patients.43 Treatment with NTZ blocks the interaction between VLA-4 and VCAM-1 on brain endothelial cells, but also results in prolonged reduction in VLA-4 expression on blood cells.44 Common side effects of NTZ are cough, difficulty with swallowing, dizziness, fast heartbeat, puffiness, or swelling of the eyelids or around the eyes, face, lips, or tongue, shortness of breath, skin rash, hives, or itching, tightness in the chest, unusual tiredness or weakness. The rare side effects of this drug include PML, pharyngitis, urinary tract infection, urticaria, cephalgia, dizziness, nausea, vomiting, arthralgia, fever, and rigidity (Table 1).
Fingolimod
Fingolimod interferes with the migration of immune cells by a mechanism that differs substantially from that of NTZ. Fingolimod decreases the number of circulating lymphocytes by targeting sphingosin-1-phosphate (S1P)-receptors (Figure 1). Fingolimod induced bradycardia and atrio-ventricular conduction block in <2% of patients.45 Other side effects were macular edema, elevated liver function tests, increased risk of respiratory tract infections, urinary tract infections, regional herpes virus infections, and hypertension. Two patients treated with 1.25 mg fingolimod daily encountered fatal herpes virus infections: disseminated primary varicella zoster infections with liver failure and herpes simplex encephalitis.46 Common side effects of Fingolimod, include diarrhea, coughing, headaches, hair loss, depression, muscle weakness, and dry and itchy skin. Bradyarrhythmia and atrioventricular blocks, macular edema, elevated liver function, increased risk of respiratory tract infections, urinary tract infections, regional herpes virus infections, and hypertension are some of the less common side effects of the drug (Table 1).
In conclusion, Alemtuzumab is a highly effective therapy for patients with active RR-MS who are either treatment-naive or who have had breakthrough disease on DMT. Multiple sclerosis treatments that selectively interfere with specific aspects of lymphocyte migration have been found to be successful. Novel treatments are now being developed that are more specific for subtypes of S1P-receptors than fingolimod, which targets 4 out of the 5 known S1P-receptors. While the list of MS therapies continues to grow, the treatment of MS remains a huge challenge. The results obtained are great in controlling inflammation while the attempts to effectively treat MS by repairing CNS damage and disabilities related to the illness are still low. The approval of new oral drugs would benefit MS patients because of the convenient routes of administration. However, further research is needed to understand the mechanisms of action, efficacy, and possible adverse events.
Acknowledgments
The author would like to thank Prof. Alexzander A. A. Asea, Deanship for Scientific Research, University of Dammam for helpful discussions and linguistic editing of the article.
Footnotes
Disclosure
The authors declare no conflicting interests, support or funding from any drug company.
- Received April 11, 2015.
- Accepted September 3, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.