Abstract
Sialidosis is a rare lysosomal storage disease caused by neuraminidase gene (NEU1) mutation and a deficiency of the enzyme neuraminidase. The aim of this study was to examine the sialidosis type 1 brain using volumetric magnetic resonance imaging (MRI), diffusion tensor imaging and functional MRI in comparison to 3 controls. The patient’s gene analysis identified compound heterozygous mutation in the NEU1 that is shown to be associated with the sialidosis type 1. In this very rarely seen case, we found volume changes in different brain structures. We found that subthalamic nucleus volumes were found to be smaller in the patient compared to the controls. Also, sialidosis type 1 had significantly smaller cerebellar volume compared with the control group. The case had higher mean diffusivity and lower fractional anisotropy values in the cerebellum and displayed abnormal functional connectivity.
Sialidosis (Neuraminidase deficiency, OMIM# 256550) is a rare autosomal recessive disease with approximate prevalence of 1/5,000,000-1/1,500,000 live births, related to neuraminidase gene (NEU1) (OMIM * 608272) pathogenic variants, leading to neuraminidase (EC 3.2.1.18) deficiency.1 Due to age of onset and severity, Sialidosis divided into 2 types; milder, late-onset, non-dysmorphic form, characterized by macular cherry-red spot, visual defects, myoclonus, ataxia, and seizures - Sialidosis type 1 and severe, early-onset, dysmorphic form - Sialidosis type 2.2 Myoclonic movements are known to have many possible causes. Special attention should be given to associated symptoms and signs in patients with ataxia and myoclonus. Progressive myoclonus has been associated with lysosomal storage diseases such as sialidosis type 1. In this report, we present brain examination results of the sialidosis type 1 case by using advanced neuroimaging techniques: volumetric magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI), and functional MRI and compare it to 3 controls. Also, this study presents a novel pathogenic variant of the NEU1 gene and describes eye findings.
Case Report
Patient information
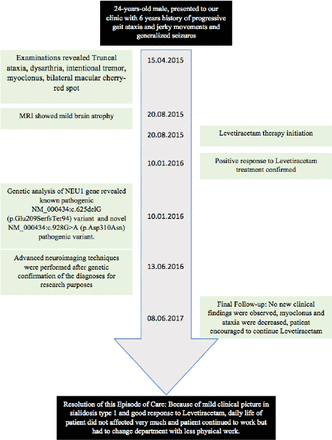
The patient, 24-years-old male, was the first child of non-consanguineous healthy parents, presented to our clinic with 6 years history of progressive gait ataxia and jerky movements. Handling timeline of this case is described in Figure 1.
Handling timeline of 24-years-old male, presented to our clinic with 6 years history of progressive gait ataxia and jerky movements.
Clinical findings
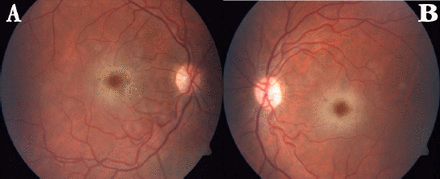
Neurological examination revealed truncal ataxia, dysarthria, and intentional tremor of the upper limbs, negative myoclonus and stimulus sensitive myoclonus in the arms. Funduscopic examination disclosed bilateral macular cherry-red spots (Figure 2). Patient also had generalized seizures.
Color fundus photography image showing ‘cherry red spot’ appearance due to whitening of the retina in the perifoveal area A) Right eye, B) Left eye
Diagnostic assessment
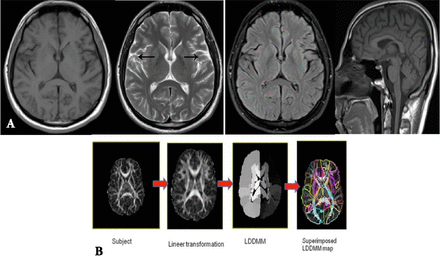
The brain MRI showed mild atrophy (Figure 3A). The patient’s genetic analysis, sequencing of the exons and intron/exon splice sites of NEU1 gene by using Sanger method, identified 2 heterozygous pathogenic variants; previously reported and known to be pathogenic NM_000434:c.625delG (p.Glu209SerfsTer94) variant and novel NM_000434:c.928G>A (p.Asp310Asn) variant.3
Neuroimaging results A) The patient’s brain MRI figures (white arrows show atrophy) with axial T1, T2, FLAIR and midsagittal T1. B) The patient’s brain DTI data.
One patient with sialidosis type 1 and 3 age-matched healthy control males were selected for the study. The eligibility criteria for control subjects were as follows: normal developmental history, normal findings in radiology and normal neurologic examination.
Subjects underwent MRI by using a 20-channel head coil, 1.5 Tesla clinical scanner (Magnetom Aera, Siemens Healthcare, Erlangen, Germany) at Radiology department. T1-weighted magnetization prepared rapid acquisition gradient-echo sequence, axial and coronal T2-weighted sequence, Blood oxygenation level dependent (BOLD) imaging and twice-refocused DTI. Participants had to stay still, eyes-closed, and remain awake during BOLD imaging.
The DTI data (Figure 3B) were analyzed with DTIStudio, ROIeditor and DiffeoMap programs. The raw diffusion-weighted images were processed using a 12-mode affine transformation of Automated Image Registration and then Large Deformation Diffeomorphic Metric Mapping.4 After normalization to the JHU-DTI-MNI ‘‘Eve’’ template, each participant’s original brain space was parcellated into 189 anatomical structures. Finally; the volume, fractional anisotropy (FA) and mean diffusivity (MD) were measured for each cerebral region for all subjects for 189 parcellated brain structures.5
The preprocessing of BOLD images was performed with Statistical Parametric Mapping (SPM8) software (The Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm8). The preprocessing steps included realignment, coregistration to MPRAGE images, normalization to a standard template, and smoothing with an isotropic Gaussian kernel of Full Width at Half Maximum 6 mm. The ROI-based functional connectivity tests were performed with the Functional Connectivity toolbox.6 In total 89 Brodmann Areas (BA) were examined using ROI to ROI analysis and functional connectivity values were obtained and voxel-wise comparisons restricted to this ROI were evaluated at p<0.005 (uncorrected). To compare the differences between sialidosis type 1 patient and controls, independent-samples t-test was performed. Analysis was conducted using IBM statistical package for the social sciences statistics 22 software with considering a p-value<0.05 statistically significant. The volume measurements of brain structures such as cerebellum, precentral gyrus (PreCG), postcentral gyrus (PoCG) and subthalamic nucleus were performed. These measurements are shown in Table 1. The volumes of the selected regions were smaller in the patient compared to the controls.
The volumes (cm3) of the selected brain structures.
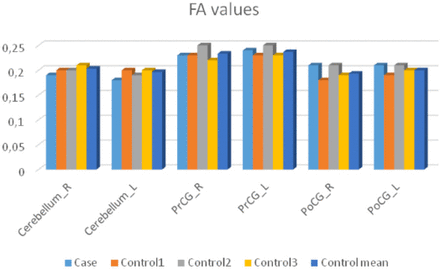
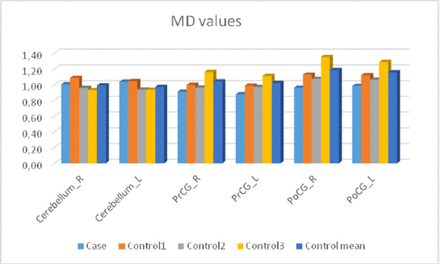
Diffusion measures for sialidosis type 1 and the control group are shown in Figure 4 and Figure 5. The patient had lower FA in the cerebellum but higher FA in the other regions. The patient had higher MD values in the cerebellum whereas lower MD values in the remaining regions (Figure 4, 5).
Fractional anisotropy (FA) values for the case and the control subjects.
Mean diffusivity (MD) values for the case and the control subjects (mm2/s).
The functional connectivity between the left anterior prefrontal cortex (BA-10L) and the right dorsal entorhinal cortex (BA-34R) was lower in the patient. The left inferior temporal gyrus (BA-20L) showed higher connectivity with the right perirhinal cortex (BA-35R) in the patient. The connectivity between right frontal eye fields (BA-8R) and right fusiform gyrus (BA-37R) among the one between BA (8R) and dorsolateral prefrontal cortex (BA-46R) were lower in the patient. The left inferior temporal gyrus (BA-20L) and the right perirhinal cortex (BA-35R) was seen higher connectivity in the patient. Also, the connectivity between BA (8R) and the left inferior parietal lobe was higher in the patient. Also, right angular gyrus (BA-39R) showed less connectivity with the left anterior superior temporal gyrus in the patient.
Therapeutic intervention
Patient had generalized seizures and was diagnosed with epilepsy which responded to Levetiracetam therapy. Myoclonus and ataxia also decreased after Levetiracetam treatment.
Follow-up and outcomes
No new clinical findings were observed during follow-up examinations, patient continued to be seizure-free under Levetiracetam treatment, myoclonus and ataxia were also decreased, patient encouraged to continue treatment. Because of myoclonus and ataxia patient was frequently falling and because of decrease in this complaints the patient’s balance problem resolved.
Discussion
We report here the clinical and the genetic findings of a sialidosis type 1 case with a novel pathogenic variant in the NEU1 gene and neuroimaging features. Genetic analysis revealed heterozygous c.625delG and heterozygous c.928G>A variants in our patient, so determination whether position of this variants is cis or trans, evaluation of family segregation needed. Further investigation revealed that father and elder brother are carriers of c.625delG and mother is heterozygous for c.928G>A variant which proves trans positioning compound heterozygosity in our patient. No clinical findings were observed in family members. Lukong et al3 previously reported homozygous c.625delG (HGMD: CD000945) frameshift variant in infant with complete absence of neuraminidase activity in the cultured fibroblasts. Reported patient was Turkish origin born as our patient, which may suggest that c.625delG variant allele frequency is higher in Turkish population than world population which explains why this variant was neither found in Exome Aggregation Consortium (ExAC) nor 1000G database. Novel c.928G>A variant in silico analysis (MutationTaster, PolyPhen and SIFT) predicted pathogenicity, this variant was not reported in 1000G and was reported as a singleton in ExAC and can be found (rs759646819) to be clinically unknown significant variant in dbSNP database. Considering all above-mentioned descriptions c.928G>A variant was classified as pathogenic according to American College of Medical Genetics (ACMG) and Genomics and the Association for Molecular Pathology, 2015 guidelines.
We revealed volume changes in different brain structures in sialidosis type 1 case. Our case showed lower volume in the right PrCG and bilateral PoCG but higher volume in left PrCG (Table 1). The patient also had significantly smaller cerebellar volume compared to the control group. He also had higher MD values and lower FA values in cerebellum. The patient had abnormal functional connectivity. Moreover, our functional MRI findings might be normal variation because of the small number of controls.
Lu et al4 recently carried out a study with 11 sialidosis type 1 patients to show cortical damage using DTI and fMRI. The MRI findings of this study suggests that the posterior parts especially occipital lobe also affected. Functional connectivity from the temporal and occipital lobes to the hippocampus and parahippocampus was decreased which evidenced with the increased MD and altered white matter integrity. These sites are the same with the atrophied regions.
The color fundus photography image shows ‘cherry red spot’ appearance due to whitening of the retina in the perifoveal area in this case. Also, optical coherence tomography (OCT) scans passing through the center of the fovea revealed hyperreflectivity in the nerve fiber layer. Kersten et al5 showed in a case with sialidosis type 1 the same abnormality in the retinal photography and spectral domain OCT. Perimacular and peripapillary retinal nerve fiber layer thickening was observed in OCT.
The sialidosis type 1 is a very rare cause of myoclonus. There are 4 forms of myoclonus which are cortical, subcortical, spinal and peripheral myoclonus types. The cortical myoclonus is the most common finding in sialidosis type 1 patients.6 Our results may contribute to the understanding of the cortical myoclonus pathophysiology.7
Patient perspective
Because of the mild clinical picture in sialidosis type 1 and good response to Levetiracetam, daily life of patient did not affected very much and patient continued to work but had to change department with less physical work after initiation of treatment.
In conclusion, described here advanced neuroimaging features of a case with sialidosis type 1 caused by a novel pathogenic variant in NEU1 gene which has not been previously reported maybe a valuable information for explanation of the etiopathogenesis of some symptoms seen in this disease.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received July 16, 2017.
- Accepted November 29, 2017.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.