ABSTRACT
Objectives: To explore the risk factors of unexplained early neurological dererioration (END) after IVT, and explore the underlying mechanisms by which these factors contribute to END onset and progression.
Methods: We performed a systematic literature search in accordance with PRISMA guidelines, utilizing PubMed, WOS, and EMBASE databases to identify all relevant studies investigating END in AIS patients who received IVT within 4.5 hours of symptom onset.
Results: Out of 2,613 reviewed records, 16 were included in this meta-analysis. The quantitative synthesis of data regarding the incidence of END in acute ischemic stroke (AIS) patients with IVT was 12% (95% confidence interval [CI], 10%-15%). Several factors were identified as significantly associated with post-IVT END, including demographic characteristics (age, male sex), comorbidities (hypertension, diabetes mellitus, atrial fibrillation), medications (antihypertensives, antiplatelets), admission parameters (hyperglycemia, elevated white blood cell count, cholesterol levels, blood pressure readings), timing of treatment, and the presence of large artery atherosclerosis (LAA).
Conclusion: Understanding and monitoring multiple factors associated with END, including other comorbidities, may achieve satisfactory results. The investigation of white blood cells’ involvement in END following AIS merits particular attention, as it may guide the development of targeted preventive medications.
Clinical research has widely confirmed that intravenous thrombolysis (IVT) administered within the effective time window is the recognized standard treatment for acute ischemic stroke (AIS).1-3 Though the initial 24 hours typically bring improvement for most patients, a significant portion either fails to show substantial recovery, or worse, experiences early neurological deterioration (END).4,5 The definition of END includes an increase of 4 points or more in the National Institutes of Health Stroke Scale (NIHSS) score within 24 hours after receiving thrombolytic treatment.6,7 The incidence of END varies from 8% to 28% of AIS patients following intravenous thrombolysis (IVT).8 Most of the poor prognosis of 3 months in patients with AIS after IVT was significantly associated with END, which elevates the risk of disability and death of stroke.9,10
The END is generally associated with the severity of stroke, reperfusion injury, cerebral edema, and symptomatic intracranial hemorrhage (sICH).4 The first 24 h after IVT is a critical period for clinical outcomes to improve or worsen. Except for definite cases such as sICH, malignant edema, and early recurrent ischemic stroke (ERIS),11 the clinical evolution of END caused by other unknown etiologies is difficult to predict. Unexplained END12 refers to neurological deterioration due to complications without any of these or other potentially identifiable etiologies (e.g., seizures after stroke), and predictors and related factors are largely unknown. Elucidating the risk factors for unexplained END within 24 hours after intravenous thrombolysis can provide a basis for clinical doctors to screen and stratify high-risk patients and achieve precise medical care during the “perioperative thrombolysis period,” which is of great significance in improving the outcome of stroke patients.
We present here a meta - analysis of the causes of END to explore the risk factors of unexplained END after IVT, and explore the underlying mechanisms by which these factors contribute to END onset and progression.
Methods
Search Strategy
All relevant prospective and retrospective case-control studies were extracted from three major databases (PubMed, Web of Science, and EMBASE), including English-language publications up to May 2023. The top search terms were ‘ischemic stroke, “thrombolytic therapy, “intravenous thrombolysis,’ ‘neurological deficit,’ ‘neurological decline,’ and ‘neurological deterioration’.
Eligibility criteria
Studies were included if they met the following criteria: (1) enrolled adult patients (≥18 years) with hyperacute AIS (<4.5h from onset); (2) provided IVT with rt-PA within the 4.5-hour therapeutic window; (3) utilized NIHSS for stroke severity assessment, documenting neurological deterioration as a ≥4-point increase at 24 hours.
Exclusion criteria consisted of: (1) application of thrombolytic therapy after 4.5 hours of onset; (2) studies with inconsistent diagnostic criteria for END; (3) studies on bridging endovascular therapy after intravenous thrombolysis; (4) no outcome statistics; (5) case reports, reviews, conference abstracts, animal trials, guideline consence, and (6) republished research.
Data extraction and quality assessment
Data were extracted using a predefined protocol by two authors (J. Li and C.L. Zhu) independently, and a third author (M.L. He) intervened if there was an objection. The extracted items included: (1) basic information of studies (i.e., the first author’s name, publication year, country, design, setting, and sample size); (2) demographics (i.e., age, sex, and body mass index); (3) stroke-related characteristics (i.e., systolic blood pressure, diastolic blood pressure, NIHSS on admission, door-to-needle time, onset-to-treatment time, stroke subtype); (4) presence of comorbidities (i.e., hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, previous stroke, smoking, drinking); (5) related medications (i.e., taking oral antihypertension, antidiabetic, antiplatelets); and (6) related laboratory tests on admission (i.e., glycemia, white blood cell count, cholesterol, triglyceride, and low-density lipoprotein).
Two independent reviewers (C.Y. Zhang and L.M. Li) assessed the methodological quality of the included studies using the Newcastle-Ottawa Scale (NOS). The total NOS score was 9, and a 7-point boundary was used to distinguish high-quality from low-quality studies.
Statistical analysis
All analyses were performed using STATA 14. The conversion of median and interquartile range (IQR) to mean and standard deviation (SD) was conducted using McGrath et al13 methodology. Data synthesis was performed using the following approach: (1) binary outcomes were analyzed using pooled risk ratios (RR). (2) continuous outcomes were assessed using weighted mean differences (WMD). (3) all estimates included 95% confidence intervals (CI). (4) model selection was based on heterogeneity: 1)random-effects model for significant heterogeneity; 2)fixed-effects model for non-significant heterogeneity; 3)heterogeneity was considered significant when I²>50% or p<0.05. In addition to the visual analysis of the fuel plot, we used the Egger test for continuous variables and the Perter test for binary variables to assess publication bias, with p<0.05. To investigate sources of heterogeneity, we performed sensitivity analyses using a sequential exclusion approach, removing studies that fell outside the 95% confidence interval (CI) of the meta-analysis results. The obtained results were compared with the analysis results when all studies were included to test the stability of the results of the meta-analysis.
Results
Search and screening results
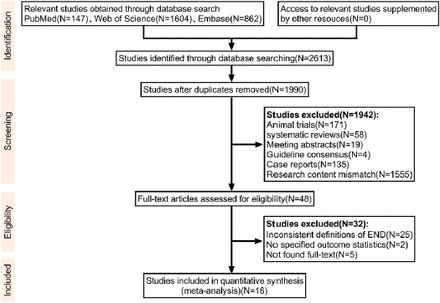
Initial database searches of PubMed, Web of Science, and EMBASE identified 2,613 potentially relevant articles. After eliminating 623 duplicates, 1,990 articles remained for screening. Title and abstract review led to the exclusion of 1,942 articles, leaving 48 articles for full-text evaluation. Ultimately, 16 studies, encompassing 58,915 AIS patients, met our inclusion criteria and provided the required outcome data. Figure 1 presents the specific screening process.
- A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart was employed to demonstrate the study selection workflow. END, early neurological deterioration.
Study characteristics and quality assessment
In the 16 studies included, 15 employed a retrospective design, and 7 were multicenter studies. The sample sizes of the included studies ranged from 74 to 50,726 patients. Among the 58,915 patients who received thrombolytic therapy in these 16 studies, 4,269 patients experienced early neurological deficits (END). The characteristics of the studies, including design, setting, sample size, age, sex, and quality scores, are detailed in Table 1. All the studies included in this analysis were of good quality. Details of the quality assessment scoring are shown in Supplementary Table 1.
- Characteristics of the included studies.
- Quality assessment of included studies using Newcastle Ottawa Scale(NOS).
Incidence of END
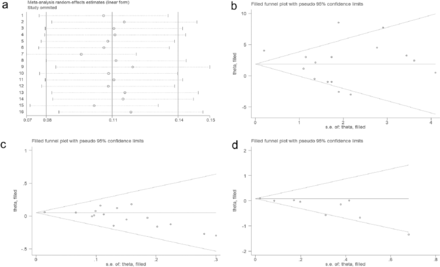
Quantitative synthesis using a random-effects model showed that the pooled overall incidence of END following IVT in patients with AIS was 12% (95% CI, 10%–15%, I2=95.88%, p<0.001 for heterogeneity, Table 2). None of the 16 included studies showed significant heterogeneity (Figure 2a).
- Random effects A) model for the incidence of END in the 16 included studies; B) estimation of the number of studies on age missing using the scissors compensation method; C) estimation of the number of studies on sex missing using the scissors compensation method; D) estimation of the number of studies on antiplatelets missing using the scissors compensation method.
- Incidence of END following IVT in AIS patients.
Predictors of END
Of the 16 studies on IVT-treated patients, 27 relevant baseline variables were included in the evaluation: age, sex, body mass index, NIHSS score on admission, door-to-needle time, onset-to-treatment time, stroke subtype according to Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, previous stroke, smoking, drinking, related medications, and related laboratory tests on admission.
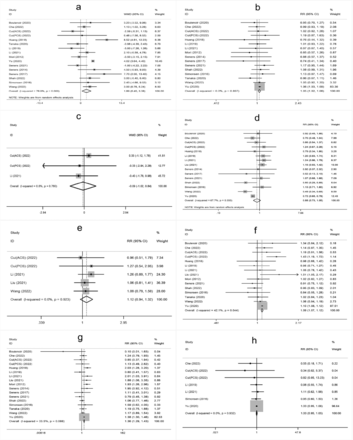
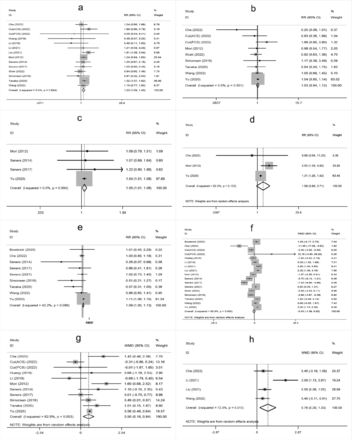
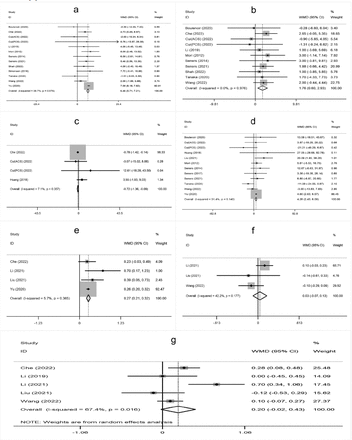
The meta-analysis showed that higher age, male sex, a history of hypertension, diabetes mellitus, atrial fibrillation, oral antihypertensive, antiplatelet use, hyperglycemia on admission, higher white blood cell count on admission, cholesterol (TC), onset-to-treatment time (OTT), systolic blood pressure (SBP), diastolic blood pressure (DBP), and large artery atherosclerosis (LAA) were significantly associated with END after IVT (Supplementary Figure 1-4).
- Supplementary Forest plot of A) Age; B) Gender (male); C) BMI; D) current smokers; E) Current drinkers; F) Hypertension; G) Diabetes mellitus; H) Hyperlipidemia. BMI - Body mass index. The solid squares represent the weighted mean differences (WMD)/the risk ratios (RRs), the horizontal lines show the 95% confidence intervals (CIs), and the diamond indicated the pooled effect size.
- Supplementary Forest plot of A) Atrial fbrillation; B) Previous stroke; C) Antihypertensives; D) Antidiabetic; E) Antiplatelets; F) NIHSS on admission; G) Glycemia; H) WBC. NIHSS, National Institutes of Health Stroke Scale. WBC - white blood cell count.
- Supplementary Forest plot of A) SBP; B) DBP; C) DNT; D) OTT; E) TC; F) TG; G) LDL. SBP - systolic blood pressure; DBP - diastolic blood pressure; DNT - door-to-needle time; OTT - onset-to-treatment time; TC - cholesterol; TG - triglyceride; LDL - low density laipoprotein.
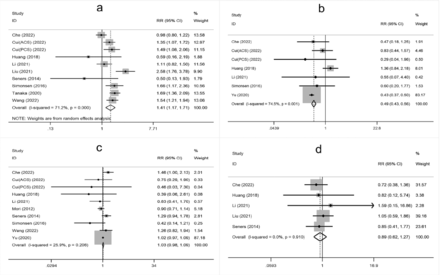
- Supplementary Forest plot of TOAST classification. A) LAA; B) SAO; C) CE; D) Other. TOAST, Trial of Org 10,172 in Acute Stroke Treatment. LAA - large artery atherosclerosis; SAO - small-artery occlusion; CE - cardio embolism; Other, stroke of undetermined.
Publication bias and sensitivity analysis
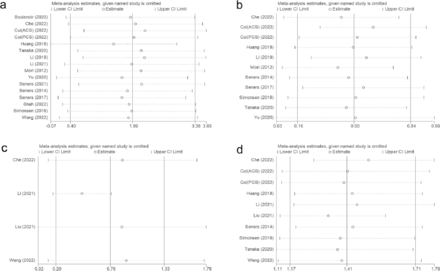
Visual funnel plots combined with the quantitative analysis of Egger’s test or Peter’s test (p<0.05) were used to detect publication bias, indicating that age, sex (male), and antiplatelets had a certain publication bias (Table 3). Based on publication bias, the scissor-compensation method was used to estimate the number of missing studies, and a quantitative analysis was performed again after filling in the corresponding number of studies. Age, sex, and antiplatelets were separately assessed using the scissor-compensation method. No significant publication bias was detected, as demonstrated by the consistency of the combined effect size, indicating robust results (Figure 2b, 2c, 2d).
- Publication bias.
A sensitivity analysis was performed to investigate the source of high heterogeneity found in predictors including age, glycemia, white blood cell (WBC) count, and LAA. The analysis showed that heterogeneity persisted even after removing the outlier study, with no significant variations detected among the remaining studies (Figure 3).
- Sensitivity analysis, A) age; B) glycemia; C) WBC; D) LAA.
Discussion
According to our meta-analysis of AIS patients receiving IVT, increased END rates were associated with male sex, histories of hypertension, diabetes mellitus, and atrial fibrillation, current use of oral antihypertensives and antiplatelets, elevated admission glucose, higher blood pressure values (both systolic and diastolic), increased white blood cell count, elevated cholesterol, prolonged onset-to-treatment time, and presence of large artery atherosclerosis. Among the above risk factors, most (i.e., hypertension, diabetes mellitus, atrial fibrillation, oral antihypertensives, antiplatelets, and glucose) have been discussed in detail in the previous meta-analysis, and we will focus on the impact of white blood cell levels on END at admission.
In Yu’s10 study, aging was associated with a higher risk of END, which was consistent with the results of our meta-analysis. The increasing lifetime risk of stroke is attributed to both an aging population and the accumulation of risk factors. Age-related neural function deterioration may be mechanistically linked to two factors: elevated brain levels of phosphorylated adenosine monophosphate-activated protein kinase (pAMPK)27 and reduced Na-K-Cl cotransporter expression.28 However, the specific mechanism requires further investigation.
The END occurred more frequently among male patients compared to females. A negative correlation exists between total testosterone levels and infarct size in men after acute ischemic stroke, during which serum testosterone levels decrease.29 Peripheral immune function is inhibited by dihydrotestosterone (DHT) in the aftermath of ischemic stroke.30 The DHT impedes post-ischemic recovery by eliminating immature neurons and reducing tissue repair capacity in ischemic regions.31 Mice that received male microbiota were significantly worse at preventing brain damage and restoring neurological function than those that received female microbiota.32 The presence of male-characteristic gut microbiota elevates systemic pro-inflammatory cytokines after ischemic stroke, while the introduction of female gut microbiota can favorably alter unfavorable stroke outcomes.32
Onset-to-treatment time (OTT) was a significant predictor of increased risk of END in our study. The longer the OTT time, the greater the likelihood of END occurrence. The analysis demonstrated a positive correlation between blood pressure levels and END incidence. Evidence from a high-quality meta-analytic study indicated that AIS patients experiencing HT face twice the risk of adverse events, including deteriorating neurological function, seizures, poor functional outcomes, and death.33 Its exact cause is unknown, but it may be related to high blood pressure, which can aggravate the hyperperfusion of brain tissue after IVT in patients with AIS. Regular adherence to antihypertensive medication effectively lowers END occurrence rates.
The atrial fibrillation identified in this analysis was in line with previous studies. Patients with atrial fibrillation who received IVT had an increased risk of neurological deterioration. This might be related to new embolic events and cerebral ischemia due to pre-existing intracardiac or arterial thrombotic ruptures. Vessel occlusions frequently occur in major arteries, characterized by poor collateral compensation and resulting in extensive infarct regions.
Large-artery atherosclerosis (LAA) was observed more frequently in patients with END. One possible explanation for this association is recanalization failure or remote migration of thrombus after IVT in large atherosclerotic cerebral infarctions. antiplatelet drugs exert protective effects against END. It has been reported that anti-platelet drug resistance in the Chinese population is associated with recurrent ischemic stroke and early neurological deterioration after acute mild ischemic stroke.34
A history of diabetes mellitus and serum glucose level on admission was associated with a higher risk of developing END. Notably, poor neurological outcome resulted from persistently high serum glucose levels following IVT. Possible mechanisms of neurologic deterioration associated with higher blood glucose levels include increased lactate production, disrupted cellular metabolism, and promotion of the formation of new infarction foci in ischemic semi-dark band tissue.35,36
In our study, cholesterol was found to be a risk factor for END, which is inconsistent with previous research. Several studies have reported that triglycerides, rather than cholesterol, are associated with death and END in AIS.37-39 This result may be due to the small number of articles on cholesterol and triglyceride levels included in our meta-analysis.
The white blood cell (WBC) count at admission was an important predictor of END in our meta-analysis. An increasing number of studies have found that the leukocyte and neutrophil counts of patients with END are increased, which shows that inflammation is a key factor in the formation of atherosclerosis and plaque rupture.40 In an experimental stroke model, the selective reduction of white blood cells after ischemia leads to a smaller cerebral infarction area.41 This suggests that infiltration of circulating white blood cells leads to microvascular blockage and amplification of toxic inflammatory mediators, which aggravates ischemic brain injury. The adhesion molecules on vascular endothelial cells and the LFA-1 and Mac-1 receptors on the surface of neutrophils recognize each other through–nd the receptor reactions. However, the molecular basis of increased neutrophil adhesion depends on increased expression and activation of the integrinβ2 subfamily CD11/CD18 on the surface of neutrophils induced by chemokines. In a septic encephalopathy model,42 chemokine (C-X-C motif) ligand 1 (CXCL1) promoted leukocyte adhesion via MAC-1 / (CD11b / CD18) binding. As a neutrophil chemokine, CXCL1 participates in inflammatory disease development, demonstrates elevated levels during inflammatory responses, possesses angiogenic properties, and facilitates tumor development. Research using a CLP mouse model demonstrated that CXCL1 neutralizing antibody treatment significantly decreased both the adhesion of rhodamine-labeled leukocytes to cerebral vasculature and the expression of ICAM-1 in endothelial cells.43 It has been speculated that the high expression of CXCL1 after stroke is an important initiating link in triggering ischemic neuronal damage. Therapeutic strategies targeting CXCL1 inhibition and ICAM-1 downregulation may represent a promising approach to prevent END by reducing neutrophil recruitment and migration to ischemic cerebral tissue. This finding is worthy of further research.
This meta-analysis has a few limitations. Most of the papers included in this study were retrospective case-control studies, which may have resulted in selection bias. Due to incomplete or unavailable data in some published studies in recent years, strict exclusion from statistical analysis may lead to the loss of useful information. Based on the inclusion and exclusion criteria, our results do not apply to patients treated with endovascular therapy or unthrombolytic therapy.
In Conclusions, END is considered a common complication of IVT in patients with AIS and seriously affects the 3-month prognosis of patients. Following intravenous thrombolysis, END was observed in 12.0% of acute ischemic stroke patients, as indicated by our meta-analysis. This meta-analysis is similar to other meta-analysis results; that is, there are many risk factors for the occurrence of END, including other complications, age, male sex, hypertension, diabetes, atrial fibrillation, major artery atherosclerosis, blood glucose level at admission, systolic blood pressure, diastolic blood pressure, antihypertensive drugs, antiplatelet drugs, time to start treatment, and cholesterol level. In addition, an important finding of this meta-analysis is that the level of white blood cells (WBC) at admission is an important predictor of END, and its mechanism of action deserves further exploration.
Acknowledgments
We would like to express our gratitude to the Neurology Department, Lianyungang Clinical College of Nanjing Medical University for their academic support. We would like to thank American Manuscript Editors (https://americanmanuscripteditors.com) for English language editing.
Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received October 23, 2023.
- Accepted February 5, 2025.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.