Abstract
Objective: To study the effects of human urinary kallidinogenase (HUK) treatment on acute cerebral ischemia (ACI) using magnetic resonance perfusion weighted imaging (MRP) methods.
Methods: In a non-randomized controlled clinical trial, 30 patients diagnosed with ACI were enrolled and divided manually into 2 groups. The experimental group, consisting of 18 participants, was treated with HUK (0.15 Perinatal Assessment Unit/day) for 7 consecutive days. The control group was treated with routine medication. The participants underwent MRP examination on the first and fourteenth day after onset. The National Institutes of Health Stroke Scale (NIHSS) score, cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and time to peak (TTP) were compared between the groups.
Results: After undergoing therapy, the experimental HUK-treated group had lower NIHSS scores than the control group (p<0.05). The CBF improved more in the HUK-treated group than in the control group (p<0.05). Additionally, MTT and TTP were shorter in the HUK-treated group than in the control group (p<0.05).
Conclusions: Human urinary kallidinogenase improves CBF and ameliorates neurological deficits. Human urinary kallidinogenase is a safe and effective treatment approach for treating patients with ACI.
Stroke is a leading cause of morbidity and mortality worldwide.1 Acute cerebral infarction (ACI) is caused by severely reduced blood and oxygen supply, most frequently due to a clot obstructing a major blood vessel. Stroke can be incredibly burdensome to patients and their families, resulting in high treatment costs, restricted social functioning, long-term disabilities, and premature death.2 Recanalization, especially thrombolysis, can significantly improve outcomes. However, hemorrhagic transformation, neurotoxicity, and a short treatment time window are major limitations of thrombolytic therapy.3,4 Human urinary kallidinogenase (HUK), a glycoprotein extracted from male urine, has been shown to promote angiogenesis, enhance cerebral perfusion, and suppress the inflammatory response in animal trials.5,6 The HUK can also improve ACI outcomes in patients.7-9 Previous studies with animal models6 found that HUK can significantly improve neurological function with few adverse effects.6 Although HUK is widely used for acute ischemic stroke in China as a state category I new drug, it is not clear by what mechanisms the drug affects human biology. In the present study, we used magnetic resonance perfusion weighted imaging (MRP) to study the effect and mechanisms of HUK treatment on ACI.
Methods
Participants
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration, and was approved by the Human Ethics Committee of Jilin University, Changchun, China. Written informed consent was obtained from individual participants. All participants involved in the present study were admitted to the Department of Neurology, and Neuroscience Center, The First Hospital of Jilin University, Changchun, China, between January 2010 and December 2011. Patients diagnosed with acute ischemic stroke underwent a systematic neurological examination and routine laboratory biochemistry test, including hepatic function, renal function, markers of myocardial injury, blood coagulation function, and blood glucose.
The inclusion criteria were as follows: (1) diagnosed with ACI; (2) CT scan confirmation of no cerebral hemorrhaging, cancer, or trauma; (3) diffusion weighted magnetic resonance imaging (DWI) identified a new ischemic lesion; (4) National Institutes of Health Stroke Scale (NIHSS) score in the range of 4 to 20. The exclusion criteria were as follows: (1) severe cardiac dysfunction, chronic liver disease, pregnancy, or hemorrhagic disease; (2) thrombolytic therapy within one week; (3) taking angiotensin-converting enzyme inhibitor orally within 24 hours; (4) recurrent stroke; (5) more than 3 days passed after stroke onset.
In a non-randomized controlled clinical trial, upon considering the cost of HUK, the patients who agreed to use the drug were assigned manually to the experimental group; the other patients were assigned to the control group. The experimental group was administered HUK intravenously (0.15 Perinatal Assessment Unit/day, Guangdong Techpool Bio-pharma Corporation, Guangzhou, China) for 7 consecutive days. Treatment was started within 3 days of stroke onset. Other anti-thrombotic treatments that could have influenced the results (for example, aspirin) were the same in both groups.
Brain MRI protocols
All participants enrolled in this study underwent MRI, magnetic resonance angiography (MRA), DWI, and perfusion weighted imaging (PWI) examinations performed on a 3.0-tesla scanner (Magnetom Expert, Siemens, Erlangen, Germany). Axial MRP sequences from the vertex to the level of the lower medulla were obtained with the following parameters: 1400 ms/32 ms (repetition time/effective echo time); 1 excitation; 128 × 125 matrix; and 5 mm/1.5 mm (section thickness/gap). The recording of images at the target and reference sites were performed by 2 neuroimaging doctors who have worked in neuroimaging for 2 years, and were blinded to the diagnoses. Disagreements between these 2 observers were settled by a third observer with 10 years’ experience in neuroimaging. The measured parameters in PWI images were cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and time to peak (TTP). The region of interest (ROI) was defined as the largest cerebral infarction on DWI. Five points were randomly selected from the ROI to measure the CBF, CBV, MTT, and TTP values, and the averages of these values were used for comparisons. All patients underwent the MRP examination on the first and fourteenth day after onset. We compared the differences in the ipsilateral and contralateral hemispheres between the experimental and control groups. Furthermore, we compared the measurements on the first and fourteenth days to calculate the recovery of blood flow.
Neurology deficit assessment
Neurological function, including motor, sensory, and other neurological function, was evaluated according to the NIHSS. All patients underwent NIHSS scoring on the first and fourteenth day after onset. Blood pressure was also monitored in all patients.
Statistical analysis
All measurements were performed by 2 independent observers and were presented as the mean ± SD. Differences between the 2 groups were assessed using Student’s t-test. Probability values of p<0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences version 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Participants
A total of 30 Chinese Han acute cerebral ischemia patients were enrolled in this study. The groups’ demographic information is listed in Table 1. Eighteen patients were enrolled in the experimental group (12 patients with cerebral artery stenosis; 6 patients with cerebral artery non-stenosis), while 12 participants were enrolled in the control group (7 patients were diagnosed with cerebral artery stenosis, 5 patients were diagnosed with cerebral artery non-stenosis).
Demographic information and NIHSS scores of acute cerebral ischemia patients.
Neurological function and blood pressure
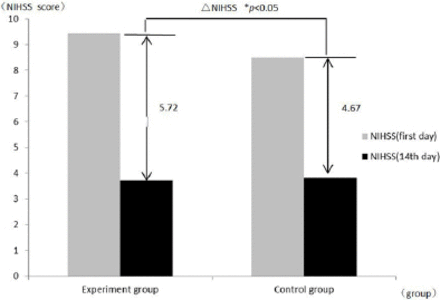
All of the participants underwent NIHSS scoring on the first and fourteenth day after onset (Table 1). We calculated the delta NIHSS (NIHSS [fourteenth day] − NIHSS [first day]). Compared with the first day after onset, the experimental group showed a significantly lower NIHSS score than the control group (p<0.05), (Table 1, Figure 1). We also found that some participants in the experimental group had a reduction of 5-10 mm Hg in blood pressure within 24 hours after treatment with HUK, but neurological function was not affected. There were no other adverse effects in the study, such as flushed face, nausea, palpitation, or vomiting.
Comparison of NIHSS scores between the HUK-treated experimental group and the control group. NIHSS - National Institutes of Health Stroke Scale; ΔNIHSS (fourteenth day) - NIHSS (first day); *p<0.05, experimental group versus control group
Magnetic resonance perfusion weighted imaging results
The measurement results for the ipsilateral and contralateral hemispheres, and the differences between the experimental and control groups are presented in Table 2 and in Figures 2 & 3. The change in CBF values between the first and fourteenth day in the ipsilateral hemisphere in the experimental group was much greater than the change in the ipsilateral hemisphere in the control group. The CBF value was increased in the contralateral hemisphere in the experimental group, while it was reduced in the control group (Table 2) (p<0.05). We also found that the difference in CBV between the first and fourteenth day in the experimental group was greater than in the control group. This difference was present in both the ipsilateral and contralateral hemispheres (p>0.05). There was a reduction in MTT between the first and fourteenth day in the experimental group, while there was an increase in the control group. This difference was present in both the ipsilateral and contralateral hemispheres (p<0.05). Furthermore, we observed a reduction in TTP in the experimental group between the first and fourteenth day in the contralateral hemisphere, while there was an increase in TTP in the contralateral hemisphere in the control group (p<0.05).
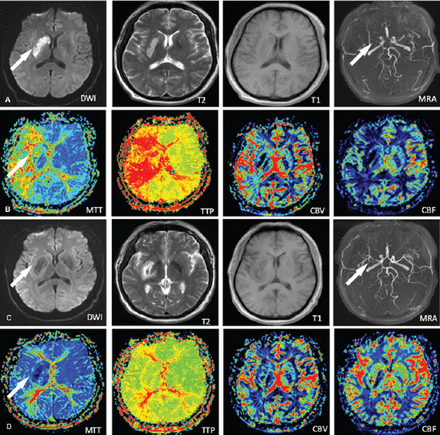
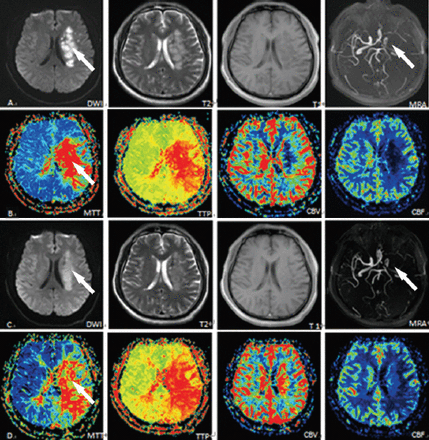
Perfusion weighted imaging measurement results from scans taken one day and 14 days after stroke onset.
Experimental group images showing: A) and B) early-stage stroke, with new infarction revealed by DWI (arrow), artery stenosis shown by MRA (arrow), and artery ischemia shown by MRP (arrow). C) and D) post-treatment images showing clear improvements in the corresponding areas (arrows). DWI - diffusion weighted magnetic resonance imaging, MRA - magnetic resonance angiography, MRP - magnetic resonance perfusion weighted imaging
Control group images showing: A) and B) early-stage stroke with new infarction revealed by DWI (arrow), artery stenosis shown by MRA (arrow), and artery ischemia shown by MRP (arrow). C) and D) post-treatment images showing little improvement in the corresponding areas (arrows). DWI - diffusion weighted magnetic resonance imaging, MRA - magnetic resonance angiography, MRP - magnetic resonance perfusion weighted imaging
Discussion
In this study, we used MRP to evaluate the microcirculation and found that HUK could improve cerebral blood flow in the lesioned hemisphere, and that it did not reduce blood flow in the contralateral hemisphere of the brain. The MRP parameters in each patient were different because all patients have different internal maladies that alter the baseline parameters required. This meant that we had to restrict our comparisons to those of the pre- and post-treatment results of each patient. After treatment with HUK, we found that the CBF and CBV increased to a much greater extent than in the control group or the contralateral hemisphere (p<0.05). However, the MTT and TTP decreased to a greater extent than in the control group or the contralateral hemisphere (p<0.05).
Compared with the first day after onset, the CBF on the fourteenth day in the ipsilateral hemisphere increased to a significantly greater extent in the experimental group than in the control group. The CBF in the contralateral hemisphere also increased in the experimental group, while it was reduced in the control group. These results indicate that antiplatelet drugs might cause blood flow to partially shift from the contralateral hemisphere to the ipsilateral (lesioned) hemisphere.
Cerebral blood volume can reflect cerebral vessel volume to some degree, which was increased significantly in the HUK-treated experimental group compared with the control group. It is well known that constriction of blood vessels can contribute to ischemia. Previous studies indicate that exogenous kallidinogenase (kallikrein) cleaves kininogen to vasoactive kinins, which binds high-affinity bradykinin B2 receptors and increases nitric oxide levels,10 thereby triggering cerebral vessel dilatation and an increase in cerebral blood flow. Therefore, we hypothesize that these vessels express B2 receptors.
In the present study, we found that 28% of participants in the experimental group showed revascularization (Figure 2) compared with 8% of controls (Figure 3), as demonstrated by MRA. Moreover, these patients’ MTT and TTP values were correlated with their CBF values, which indicates that their cerebral arteries were occluded suddenly, and that the tissue did not receive blood perfusion from other vessels. The MTT, a sensitive parameter reflecting brain tissue hypoperfusion, can be used to assess degree of ischemia.11 Therefore, our results indicate that the vascular endothelial cells of the occluded arteries (which express kallidinogenase-activated B2 receptors) were ischemic. As a result of kallidinogenase treatment, the occluded vasculature in the experimental group dilates substantially (more than in the control group), promoting recanalization.
The MTT in the experimental group was reduced after treatment, while it was increased in the control group. We also discovered that CBF in the experimental group was increased, while CBF in the control group was increased in the ipsilateral (infarcted) hemisphere and reduced in the contralateral hemisphere. Therefore, we infer that blood flow in the infarcted region can be restored by collateral or new vessels, rather than by blood stealing. Some studies5 have shown that treatment with exogenous kallikrein significantly increases vascular density as well as vascular number after cerebral infarction. In this case, the new endothelial cells may produce various vascular growth factors to contribute to neurological functional recovery after stroke.5 These previous findings are supported by our results. Furthermore, we discovered exudation around the infarction, which may be due to leaking by the newly-formed vessels.12 These newly formed vessels likely contribute to the recovery of blood perfusion following treatment with exogenous kallikrein after stroke.
The present study illustrated the mechanisms of action of HUK in the treatment of human ACI. Furthermore, our study is unique in showing that HUK ameliorates neurological deficits by improving quantitative MRP measures. The limitation of this study is that all the measurements were obtained using MRP alone, without the use of biopsy. However, MRP is a sensitive method to examine the metabolism of cerebral tissue, and can be used for evaluating brain metabolism.
In conclusion, using MRP, we found that HUK ameliorates neurological deficits in ACI by improving CBF. Given that our data is from a preliminary study, the next step would be to see if the results could be confirmed in a study with a larger sample size. Although further study is required, our results suggest that HUK may be a safe and effective drug for augmenting CBF, and improving outcomes in patients with ACI.
Acknowledgment
We thank the radiologists Hong-Wei Zhou, Ting-Ting Yuan, and Dan Tong for collecting the images.
Footnotes
Disclosure
The authors declare no conflicting interests, support or funding from any pharmaceutical company.
- Received September 10, 2015.
- Accepted December 23, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.