Abstract
Objectives: To determine nerve conduction studies (NCS) reference data for motor nerves and F-waves in the upper and lower limbs of healthy subjects in Saudi Arabia.
Methods: This is a cross-sectional study conducted between May 2015 and June 2019. Healthy subjects without neurological or systemic diseases were recruited. Motor NCS were performed following a standard protocol. Pearson correlations were employed between NCS parameters and age, gender, height, weight, and body mass index. Reference data were generated using the percentile method.
Results: A total of 127 subjects were recruited for the upper limb studies and 137 for the lower limb studies. Quantile regression models were generated to estimate compound muscle action potential amplitude (adjusted for age), as well as F-wave minimal latency (adjusted for height). The estimated reference limits of distal motor latency (ms) and conduction velocity (m/s) for the different nerves were, respectively, 3.7 and 50 for the median nerve, 3.3 and 50 for the ulnar nerve, 5.8 and 40 for the tibial nerve, and 5.0 and 40 for the fibular nerve.
Conclusion: The reference data for motor NCS parameters and F-wave minimal latency are generally comparable with those of Western countries. However, minimal differences were observed. The underrepresentation of the older age group warrants future studies.
Nerve conduction studies (NCS) are an integral part of the assessment of most patients with peripheral nervous system (PNS) disorders. Similar to other laboratory tests, interpretation of NCS requires differentiating between normal and abnormal test values. The spectrum and distribution of normal NCS values can be derived from a sample of healthy subjects that represents the targeted population. However, NCS have a wide range of normal test values that have some overlaps with NCS values of patients with a PNS pathology, which renders the unequivocal distinction between normal and abnormal NCS values difficult. Thus, the term reference, rather than normative, data has been suggested to guide interpretations of NCS results.1,2 Most neurophysiologists rely on reference data from textbooks or the literature, rather than generating their own data.3 A pitfall of most of the published reference data studies has been the lack of methodological and statistical standards.3 Therefore, the American Academy of Neuromuscular and Electrodiagnostic Medicine (AANEM) formed the Normative Data Task Force (NDTF), which has developed a set of criteria for evaluating the published NCS reference data.3 After reviewing more than 7500 articles, only 10 met all the NDTF criteria, including one article on each of the 11 routinely studied nerves,4 except for the superficial fibular nerve since none of the reviewed articles on this nerve met all NDTF criteria.4 This indicates that there is a general lack of rigorous NCS reference data. Thus far, we are not aware of any NCS reference data that meet the previously reported NDTF criteria using a Saudi Arabian study population.This study sought to collect NCS data from healthy adult participants and generate reference data for the most commonly performed NCS studies. In this article, we present reference NCS data for motor nerves and F-waves in the upper and lower limbs.
Methods
Participants and setting
The study was conducted at King Saud University Medical City (KSUMC), Riyadh, Kingdom of Saudi Arabia between May 2015, and June 2019. We included healthy Saudi subjects aged 18 years or older. The study exclusion criteria were current or history of any neurological disease, diabetes mellitus, vitamin B12 deficiency, thyroid disorder, malignancy, renal impairment, hepatic impairment, vasculitis, connective tissue disease, persistent paresthesia or numbness, exposure to neurotoxic agents (e.g., alcohol, chemotherapy, methotrexate), or bariatric surgery. For participants above 50 years of age, we performed neurological examinations and excluded those who had absent vibration at the great hallux or impaired pinprick sensation distally. We recruited the participants from clinic waiting areas and included patients’ watchers, hospital personnel, and medical students. Because the KSUMC is a tertiary hospital and accepts referrals from rural areas, we focused on recruiting people accompanying their family members (excluding those with hereditary disorders or a consanguineous spouse) from outside the city, as well as people from different Arabic tribes.
NCS protocol
In our laboratory, NCS are performed following the standardized techniques published elsewhere.5,4,6 All NCS were performed by a trained technician with more than 20 years of experience and reviewed for quality control by Drs. MHA and NMK. The belly-tendon method was used to record compound muscle action potentials (CMAPs) of the abductor pollicis brevis (median nerve), abductor digiti minimi (ulnar nerve), extensor digitorum brevis (fibular nerve), and abductor hallucis brevis (tibial nerve). The median nerve was stimulated at the wrist between the tendons of the flexor carpi radialis and palmaris longus and proximally over the brachial artery pulse in the antecubital fossa. The ulnar nerve was stimulated at the wrist lateral to the tendon of the flexor carpi ulnaris. The fibular nerve was stimulated distally in the leg just lateral to the tibialis anterior tendon, posteroinferior to the fibular head (FH), and above the FH just medial to the tendon of the biceps femoris. The tibial nerve was stimulated distally posterior to the medial malleolus and proximally in the midpoint of the popliteal fossa. The distance between the stimulating cathode and the recording electrode was maintained at 7 cm for the median (measured in a hockey stick-shaped line) and ulnar nerves and at 8 cm for the tibial and fibular motor nerves. The below-elbow stimulation site of the ulnar nerve was 4 cm distal to the medial epicondyle. The distance between above- and below-elbow stimulation sites of the ulnar nerve was maintained at 10 cm, measured in a curve with the elbow flexed at 90°and the arm abducted at an angle of 45°. The distance between above- and below-FH stimulation sites of the common fibular nerve was maintainedat 8 cm. The hand temperature was maintained at ≥32°C, and the foot temperature was maintained at ≥30°C. All motor NCS parameters were computed after achieving a supramaximal stimulation, except for the tibial nerve at the popliteal fossa, where the supramaximal stimulation was sometimes hampered by pain and technical factors. For the F-wave recording, the cathode was applied 7 cm proximal from the median and ulnar recording electrodes and 8 cm proximal from the tibial and fibular recording electrodes. A minimum of 10 F-waves was obtained with supramaximal stimulation to allow a more precise estimation of the minimal latency (ML).
Instrument setting
The NCS were performed using Nicolet Viking version 11.1 (VIASYS Healthcare Inc., USA). Low- and high-frequency filters were set at 2 Hz and 10 kHz, respectively. Sweep speed was set at 5 milliseconds per division (ms/div) for motor nerves and at 10 ms/div for F-waves. The gain was set at 2 millivolts (mV)/div for motor nerves and at 200 microvolts (µV)/div for F-waves.
The study was approved by the Institutional Review Board at KSUMC. All participants signed informed consent forms.
Analysis
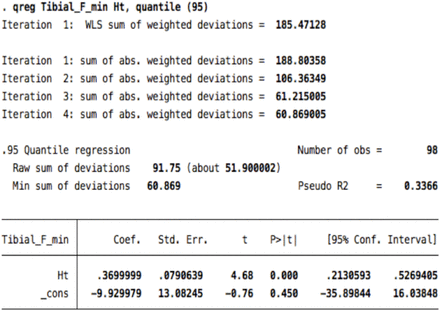
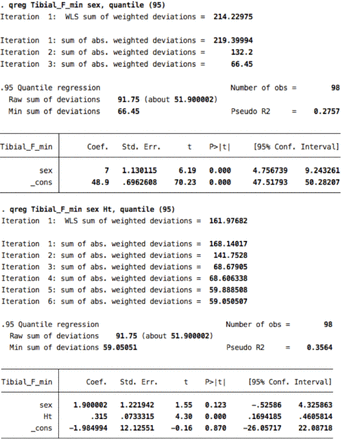
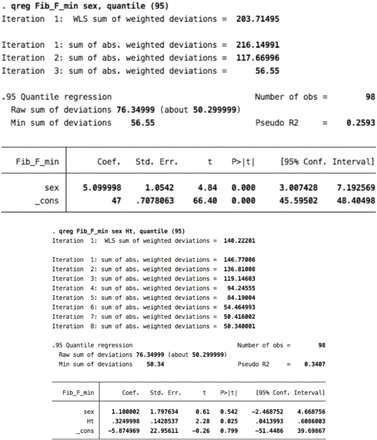
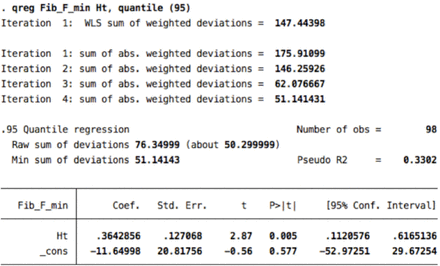
Data were summarized using descriptive statistics. Correlations between age, gender, height, weight, and body mass index (BMI) and CMAP amplitude, distal motor latency (DML), conduction velocity (CV), and F-wave ML were assessed using Pearson correlations. We log-transformed the data on NCS parameters to avoid the possibility of encountering a negative percentile value.7 We then conducted quantile regression analyses to identify the covariates that had a significant contribution to the variance of the NCS parameters. The purpose was to determine the covariates that were significantly associated with the values of the NCS parameters of related nerves to adjust for when generating reference data; for example, BMI should be adjusted for as a covariate only if an association is observed with the median, ulnar, and radial sensory nerves. This is because those with inconsistent significance across NCS parameters of related nerves may have been influenced by numerical artifacts rather than by variations in nerve biology.2 P-values<0.05 were considered statistically significant. We adjusted our reference data for age, when appropriate, as recommended by the NDTF.3 For the generation of the reference data, we considered only covariates that would result in clinically relevant differences independent of age. Finally, we computed the reference data using the most extreme percentile at which convergence of the quantile regression model was observed. The 95% confidence intervals (CIs) for these percentiles were generated to allow estimating the upper and lower bounds of the reference data, as appropriate. F-wave ML was determined using the raw data with the same method. Data were analyzed with the Stata software version 12 (Stata Corp., College Station, Texas, USA).
Results
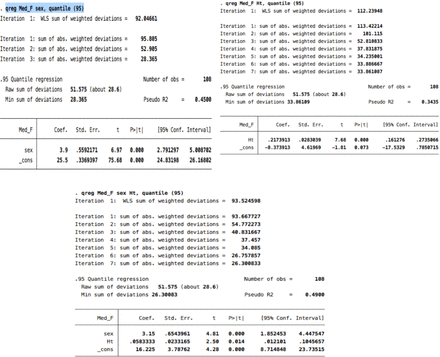
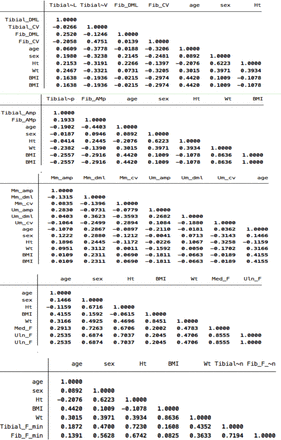
Motor NCS were performed in the upper and lower limbs in 127 and 137 participants, respectively. However, the number of participants varied for each nerve. Subjects who dropped out before the study was completed gave their informed consent for using the data that had already been collected. Table 1 shows the characteristics of the study participants. A summary of motor NCS reference data is presented in Table 2. Table 3 shows the reference data for motor NCS parameters and F-waves at the most extreme percentile that could be reliably determined. The correlations between the covariates (gender, height, weight, and BMI) and CMAP amplitude, DML, and CV were generally weak (Appendix 1). Therefore, and because age was not correlated with DML and CV, reference data for these parameters were generated for all participants pooled together using the 97th and 3rd percentiles, respectively. For CMAP amplitude,quantile regression was employed, using age as a covariate, to generate reference data at the lowest percentile that demonstrated statistical significance (Appendix 2). However, we did not find a statistically significant quantile regression model for the tibial CMAP amplitude at the 10th percentile or at even more extreme percentiles. Hence, data from all subjects were combined to calculate the reference limit for this nerve.
Characteristics of the study participants.
Summary of the motor NCS values in the upper and lower limbs.
Reference values for motor NCS parameters in the upper and lower limbs.
For motor NCS parameters, in which age contributed significantly to the corresponding prediction model, we determined reference data for age values of 20, 40, and 60 years, as shown in Table 3. The regression coefficients generated in the quantile regression model at the 3rd percentile can be used to estimate the log (predicted CMAP amplitude) for other age values. For example, the predicted 3rd percentile of the median CMAP amplitude for a 50-year-old subject would be estimated as follows:
log (median CMAP amplitude) = β0 + β1 × age
where β0 is the constant coefficient and β1 is the coefficient for age:
= 2.129 + (–0.004) × 50
= 2.129 – 0.2
= 1.929
Hence: Median CMAP amplitude = exp (1.929) = 6.9 mV. The predicted lower limit of the normal CMAP amplitude at the 3rd percentile can also be estimated using β0 and β1 at the lower bound of the 95% CI (Table 3). A comparison of the data from subjects aged 60 years with those from subjects aged 20 years shows that the effect of age was most prominent on the peroneal CMAP amplitude, which was decreased by 78-84%. The impact of age on the median and ulnar nerves was less, showing only a decrease of 15-27% (Table 3).
The correlation coefficients between F-wave ML and height were 0.67 for the median nerve, 0.70 for the ulnar nerve, 0.72 for the tibial nerve, and 0.67 for the fibular nerve. The correlation coefficients between F-wave ML and male gender were 0.72 for the median nerve, 0.68 for the ulnar nerve, 0.47 for the tibial nerve, and 0.56 for the fibular nerve (Appendix 1). The F-wave reference data are presented in Table 4.
Reference data for F-wave minimal latencies determined at the 95th percentile.
Discussion
This is the first study to provide reference data for the most commonly performed motor NCS in Saudi Arabia. The cut-off points we estimated provide general guidance to neurophysiologists and neurologists when interpreting NCS data in Arab populations. We also determined the 95% CI at the respective percentile for each nerve. It is left to the physician’s clinical judgment to use the lower bound of the 95% CI as a more conservative reference limit for CMAP amplitude and CV, and the upper bound of the 95% CI as a more conservative reference limit for DML. This latter approach may be used to increase the specificity and decrease the number of false-positive results.
A sample size of ≥100 is deemed necessary to reliably estimate reference data at the 2.5th and 97.5th percentiles.3 This supports the reliability of the percentile estimates in this study as the number of participants exceeded 100 for all recorded motor NCS parameters except for the F-wave. The F-wave ML was, however, estimated at the 95th percentile. Conceivably, using cut-off points at the more extreme percentiles would increase the specificity of the test at the expense of its sensitivity.7 Therefore, the clinical context should be considered when interpreting NCS reference data, and comparisons with the normal side are necessary, particularly when an NCS value in the symptomatic side is within the low normal range.
In general, our reference values are comparable with those of previous studies that met the NDTF standardized criteria;2,4,7-11 however, there were a few differences that will be discussed below. The ulnar motor nerve CV across the elbow was 52 m/s, the CV slowing in the across-elbow segment compared to the forearm segment was 8 m/s, and the percentage of CMAP amplitude drop across the elbow was 11.3%. The corresponding values reported by Buschbacher were 43 m/s, 15 m/s, and 16%, respectively.11 Both studies used the same technique for positioning the elbow, the same stimulation sites, and the same distance across the elbow. Regarding the fibular motor nerve, we observed a DML of 5.0 ms, the CV across the fibular head was 43 m/s, the slowing of CV across the fibular head segment compared to the leg segment was 5 m/s, and the drop in CMAP amplitude across the fibular head was 14.5%. The correspondent values reported by Buschbacher were 6.5 ms, 42 m/s, 6 m/s, and 25%, respectively.9 The DML for the median and ulnar nerves in this study was obtained with a distal distance of 7 cm, whereas the distance used in previous studies was 8 cm. After adjusting for the 1-cm difference, the DML for the median and ulnar nerves increased to 4.3 and 3.8 ms, respectively, and these values are comparable with those obtained in previous studies (4.5 and 3.7 ms, respectively).7,8,11 Contrary to findings from other studies,7,10 age was not a significant contributor to the variance in tibial CMAP amplitude. This discrepancy was also observed between the nerves in our study; for example, age was significantly associated with fibular, median, and ulnar CMAP amplitude, although its contribution to the variance was minimal in the latter 2 nerves.
These differences might be explained by the fact that our cohort was younger than that reported by Buschbacher.8-11 More specifically, we had a lower number of participants older than 50 years, which might have hindered a stronger association between NCS parameters and age. In addition, ethnic and environmental factors may also have an effect. Unlike in previous studies, our cohort consisted exclusively of Arabs. Literature review revealed no previous studies have investigated the differences in NCS parameters between Arabs and other ethnic groups. Fong et al. investigated differences in NCS parameters between three healthy Malaysian ethnic groups and found that on average, Indians have a slower sensory and motor conduction velocity, as well as higher sensory nerve action potentials and CMAPs amplitudes, in several nerves compared with Chinese and Malays.12 The authors speculated that their findings could be related to differences in skin thickness, digits circumferences, nutritional status, occupation, or genetic variations in the structure and function of the nerve.12
Height contributed significantly to the prediction model of F-wave ML, and although gender also showed significant associations with F-wave ML, its regression coefficient was attenuated to an insignificant value after adding the parameter height to the regression model. An exception was for the median F-wave ML, whereby males had a 3 ms longer median F-wave ML than females. This mild difference is not clinically significant and may have resulted from a numerical artifact especially that it was not observed for the ulnar F-wave ML (Appendix 2). In this study, males were significantly taller than females (p < 0.001) by a mean value of about 12 cm, which explains the multicollinearity between gender and height. Despite the difference in statistical analysis, the F-wave ML values in this study appear to be slightly shorter than those reported by others, especially for the tibial and fibular nerves.7,13–16 However, we believe that this difference is accounted for by the shorter mean height of our participants.
This study has some limitations. First, we recruited our cohort of healthy subjects from within the hospital, which may not give an accurate representation of the general population. However, obtaining NCS reference data from the general Saudi population through random sampling would have been an arduous task, if not impossible. The study was discontinued because of very slow recruitment. Recruitment of healthy subjects for the test was a challenging part of this study due to several factors including fear of discomfort, unwillingness to expose body parts especially the lower limbs in female subjects, the duration of the procedure (30 min) considered long by some individuals, and the lack of incentives. Logistical challenges, such as staff availability during summertime, also hindered the recruitment process. A small number of participants withdrew before the study was completed either because of time constraints or because they considered the test uncomfortable. Additionally, we had difficulties recruiting people older than 50 years of age who were completely healthy. This shortcoming of the study merits caution and clinical correlation when using our reference data for older individuals. Apart from the underrepresentation of elderly in our cohort, the NCS procedure was performed following the standards of the NDTF, and—as long as the technical factors are standardized—the reference data herein provide guidance for the interpretation of NCS to be used by clinicians in the region.3,17
References
* References should be primary source and numbered in the order in which they appear in the text. At the end of the article the full list of references should follow the Vancouver style.
* Unpublished data and personal communications should be cited only in the text, not as a formal reference.
* The author is responsible for the accuracy and completeness of references and for their correct textual citation.
* When a citation is referred to in the text by name, the accompanying reference must be from the original source.
* Upon acceptance of a paper all authors must be able to provide the full paper for each reference cited upon request at any time up to publication.
Sample references are available from: http://www.nlm.nih.gov/bsd/uniform_requirements.html
Appendix 1 Correlations of age, sex, height, weight, and BMI with motor nerve action potential amplitude, distal latency, and conduction velocity.
Appendix 2 Quantile regression analysis output.
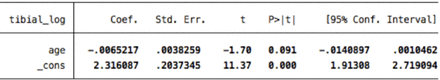
1. Tibial (2nd percentile)

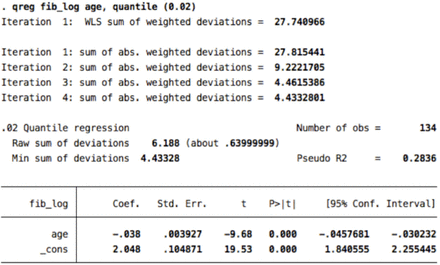
2. Fibular motor (2nd percentile)

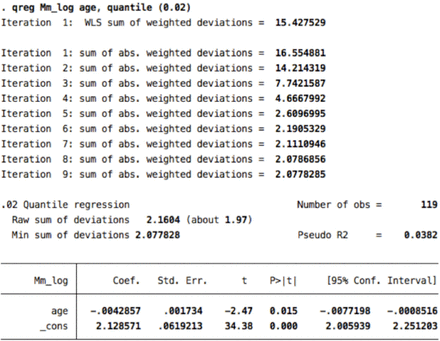
3. Median motor 2nd percentile

4. Ulnar motor: age was not a significant predictor at 2nd, or 3rd percentiles 5th percentile

5. Tibial F-minimal latency


6. Fibular F- minimla latency


7. Median F wave minimal latency

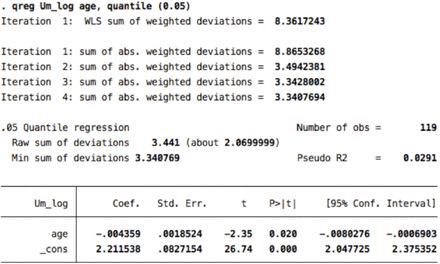
8. Ulnar F- minimal latency

Footnotes
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
- Received September 27, 2019.
- Accepted October 14, 2019.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.