Abstract
Objectives: To assess the real-world tolerability of teriflunomide in multiple sclerosis (MS) patients from a large Canadian MS Centre of Care to determine whether previously treated (PT) patients have different tolerability thresholds than treatment-naive (TN) patients, leading to differing discontinuation rates.
Methods: This non-interventional, single-center, retrospective chart review examined all patients who were prescribed commercial teriflunomide between July 2014 and May 2015 at the MS Clinic in the Ottawa General Hospital and Research Institute, Ottawa, Canada.
Results: A total of 119 patient charts were reviewed (29 TN and 90 PT). Overall, 19 (15.9%) patients discontinued teriflunomide after a mean treatment duration of 35 weeks. The most common reason for discontinuation was side effects in 8 patients (42%).Discontinuation due to intolerability alone occurred in 13 patients. The number of discontinuations was not sufficient to demonstrate a statistically significant difference between TN and PT patients (p=0.1).
Conclusions: This retrospective chart review provides some evidence about the real-world tolerability of teriflunomide. Discontinuations were low overall and consistent with previously reported clinical trial data. There was no significant difference in discontinuation rates between patients in the TN and PT groups. We believe that teriflunomide is a safe and well-tolerated oral alternative to injectable therapies.
With the expanding treatment options for multiple sclerosis (MS), the choice of a specific disease-modifying treatment (DMT) becomes more complicated. The choice of a DMT should consider in consideration the risk-benefit profile in addition to convenient route of administration. Because of the evolving landscape of DMTs for MS treatment, the choice for DMT in treatment-naïve patients is challenging. On the other hand, It is not uncommon that MS patients who are on self-injectable DMT express the desire of changing DMT, although physician recommendations for specific DMT remain highly valuable. Patients transitioning from a previous DMT may exhibit different thresholds compared with treatment-naïve patients, in which case discontinuation rates may be different. A patient with experience on a previous DMT may also have different expectations after switching to another therapy.
The DMTs are classified as a first- or second-line based on clinical efficacy and safety profile. There have been only a few head-to-head comparative investigations assessing the efficacy of one treatment over the other, especially regarding efficacy. Because the various DMTs not only have different mechanisms of action, they also exhibit varying toxicity. In the absence of true comparative data to help judge the efficacy of one DMT over another, decisions regarding treatment options are weighed heavily on patient preference, the experience of the treating physician, and expected patient tolerability.
Teriflunomide is a once-daily oral treatment option for adults with relapsing forms of MS (RMS), supported by a large clinical trial dataset from placebo-controlled, randomized trials. The 14 mg dose had the most consistent and significant effect on the 3 key measures of MS disease activity: risk for sustained disability progression; annualized relapse rate (ARR); and various measures of magnetic resonance imaging activity.1-3 It also has a satisfactory safety profile with regard to all currently available oral therapies, with no life-threatening side effects. The most common reported adverse effects include alopecia, nausea, increased alanine aminotransferase (ALT) levels, paresthesia, back pain, and diarrhea.4,5
The results of the first head-to-head clinical trial comparing teriflunomide treatment with interferon (IFN) b-1a 44 µg subcutaneously in subjects with RMS (TENERE) have been published. Treatment failure which was the primary end point in this study, was defined as either the first confirmed clinical relapse or discontinuation of the treatment permanently regardless of the reason.4 No statistical superiority in time to failure was observed when comparing the teriflunomide-treatment groups with the IFNb-1a group. However, there was no statistical difference in ARR between teriflunomide 14 mg and IFNb-1a. Although the primary endpoint of this superiority study was not met, there were positive outcomes for teriflunomide, especially for secondary endpoints of patient-reported quality-of-life measurements.6
The aim of the present study was to assess the safety and tolerability of teriflunomide in a real-life clinical setting. Our hypothesis was that patients transitioning from a previous DMT may exhibit different tolerability thresholds than treatment-naïve patients, in which case discontinuation rates may be different. Experience on a previous DMT may also lead to different expectations after switching to another therapy. One of the distinguishing advantages of teriflunomide compared with other DMTs is the availability of an accelerated elimination (washout) protocol. We also examined the frequency and reasons for patients choosing to wash out. The present study aimed to investigate the most important factors that affect patient tolerability to teriflunomide, and whether treatment-naïve MS patients exhibit better tolerability to and satisfaction with teriflunomide than patients switching from another first-line DMT.
Methods
This investigation was a non-interventional/single-center, retrospective study chart review, conducted at the Multiple Sclerosis Clinic at the Ottawa General Hospital and Research Institute, Ottawa, Canada. This study was approved by the Ottawa Health Science Network and Research Ethics Board. Given the retrospective nature of the study and the use of anonymized patient data, requirements for informed consent were waived. Patient inclusion criteria were as follows: age ≥ 18 years; RMS; and first exposure to teriflunomide or switch from another approved DMT. Exclusion criteria were as follow : Primary or secondary progressive MS and being on experimental therapy. The study measured the following outcomes: discontinuation rate (binary variable [yes versus no]); need for washout in patients who discontinued (binary variable [yes versus no]); reasons for discontinuation (side effect[s], patient preference, failure of treatment); and reasons for washout (pregnancy, side effect[s], patient request, escalation of therapy) were collected. Additionally, demographic information including such as age and gender were also collected.
Results
All charts of patients who were taking commercially available teriflunomide at any time (excluding experience in clinical trials) were reviewed (n=205) from July 2014 until May 2015. Of these, 119 patients fulfilled the study criteria and were included in the analysis. The most common reason for patient exclusion was participation in a previous clinical trial, either teriflunomide-related or other clinical trials. Of the 119 patients included in the review, 90 were switched from another DMT, 59% of whom were exposed to 1 DMT, and 41% had tried >1 DMT. Twenty-nine patients were treatment-naïve and were never on any DMT in the past.
Baseline clinical characteristic and demographic data are summarized in Table 1. As expected, patients switching to teriflunomide experienced a longer mean disease duration and a higher mean Expanded Disability Status Scale (EDSS) score. Nevertheless, there were no statistically significant differences between the 2 groups.
Baseline demographic and clinical characteristics.
Tolerability and adverse events
No serious adverse events were reported during the chart review, except for one case of cholestatic jaundice. At least one adverse event was reported by 49.1% of patients. Among the most commonly reported adverse events were hair thinning or decreased hair density (35.3%), diarrhea (17.6%), followed by nausea (15.1%).
Liver enzyme levels were measured at baseline before starting teriflunomide, at 4-6 weeks, and at 6 months of treatment. There was a slight increase in the mean ALT level 6 weeks after teriflunomide administration. All but one of the 119 patients in this chart review did not exhibit an increase in ALT level ≥1 times the upper limit of the normal (ULN) at 6 weeks, or at 6 months. One patient developed cholestatic jaundice 2 months after starting teriflunomide and liver enzyme (ALT and aspartate aminotransferase) levels were >4 times the ULN, which improved after drug discontinuation and elimination using cholestyramine.
Mean reductions in neutrophil (≤0.8x109/L) and lymphocyte count (≤0.2x109/L) at 6 weeks relative to baseline values were small in magnitude, and no further reductions were observed 6 months later. Ten percent of the patients had neutropenia below 1.7×109/L at 6 weeks, which remained virtually the same at 6 months. One patient, who discontinued teriflunomide after one year because of leukopenia (white blood cell [WBC] count 2.5×109/L, and neutropenia 0.9×109/L), had a borderline low WBC count at baseline (3.7×109/L).
Discontinuation rate
The discontinuation rate in the present study was 15.9% (n=19). The most common reason for discontinuing teriflunomide was side effects in 8 patients (42%). Gastrointestinal side effects (i.e., diarrhea and nausea) were the most commonly reported reason leading to treatment discontinuation. The second most common reason for teriflunomide discontinuation was breakthrough disease activity and escalation to a second-line DMT (e.g., natalizumab, fingolimod, alemtuzumab) in 6 patients, followed by patient choice in 5 individuals (26%).
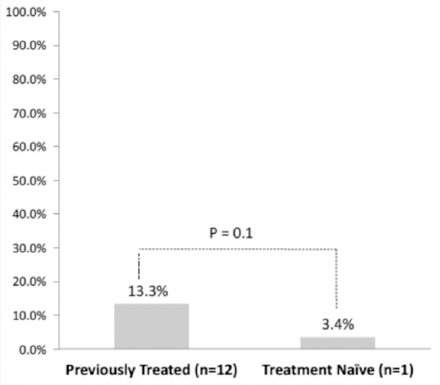
The premise was that treatment-naïve and switch patients may have different tolerability thresholds for side effects. Accordingly, an analysis was performed to compare those switching because of side effects (or patient choice) only and not those who switched because of disease progression. Tolerability was the main outcome measure and, therefore, switching or discontinuation because the physician believed disease progressed had occurred were not considered to be reasons directly related to tolerability. Discontinuation due to intolerability alone occurred in 13 patients: 1 from the treatment-naive group and 12 from the previously treated group. The number of discontinuations was not sufficient to demonstrate a statistically significant difference between the treatment-naïve and previously treated groups (p=0.1) (Figure 1). Numerically however, more previously treated patients were intolerant to teriflunomide than treatment-naïve patients, which led to treatment discontinuation.
Difference in discontinuation rates between previously treated and treatment-naïve patients.
Discussion
The aim of our study was to assess the tolerability of real-life patients to teriflunomide, and to determine whether treatment-naïve versus treated patients, who previously switched from another DMT, would exhibit different discontinuation rates due to intolerance. This retrospective chart review provides some evidence about the real-world tolerability of teriflunomide. Overall, discontinuations were low, which is consistent with what has been reported in the clinical trial data.
The most frequent adverse event reported by patients in our review was decreased hair density or hair thinning (35.3%), which was higher than was found in the TOWER2 (13%) or TEMSO3 trials (13.1%), which could be over-reported because patients were told to expect hair loss. However, hair thinning was transient and improved over time. The second most common reported side effect was diarrhea followed by nausea, both of which are quite tolerable.
Only 6 of 119 (5%) patients overall discontinued treatment due to breakthrough disease activity (almost 6% in the TEMSO study extension), and all were from the previously treated group whose disease duration was nearly 2 that of treatment-naïve group. There was no significant difference in discontinuation rates between the patients in the treatment-naïve and the previously treated groups; however, numerically more previously treated than treatment-naïve patents were intolerant to teriflunomide.
In conclusion, this simple chart review study provides some evidence about teriflunomide tolerability in a real-life clinical setting. Our observations are in accordance with the results of the TOWER and TEMSO trials. With advances in MS treatment and the availability of several DMTs, choosing which may be the best for treatment-naïve, newly diagnosed patients can be challenging. Teriflunomide is an oral treatment option, which provides a convenient administration route, good tolerability, and a very reasonable safety profile. In our opinion, teriflunomide is a safe oral alternative to injectable therapies (glatiramer acetate, interferon), which also have an excellent safety profile but have some limitations in terms of tolerability (especially long term) and patient acceptability.
Footnotes
Disclosure. Prof. Mark Freedman has received honoraria or consultation fees from Bayer Healthcare, Biogen-Idec, Chugai, EMD Canada, Genzyme, Novartis, Sanofi-Aventis and Teva Canada Innovation. He is a member of the following company advisory boards, board of directors or other similar group for Bayer Healthcare, Biogen-Idec, Hoffman La-Roche, Merck Serono, Novartis, Opexa and Sanofi-Aventis. He is a participant in Genzyme’s company-sponsored speaker’s bureau. Hind Alnajashi had received an educational grant from Sanofi-Genzyme, Foziah Alshamrani has no financial disclosures or conflicts of interest to declare.
- Received January 2, 2018.
- Accepted April 25, 2018.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.