Abstract
Objectives: To assess and compare the diagnostic accuracy, sensitivity and specificity of perfusion-weighted imaging (PWI) and positron emission tomography (PET) in distinguishing between treatment-related changes and tumor recurrence.
Methods: We carried out a systematic review of PubMed, Embase, Web of Science, the Cochrane Library, and CINAHL databases from database inception until August 2021 for pertinent articles. Particular inclusion and exclusion criteria were applied to select the eligible studies. The Quality Assessment of Diagnostic Accuracy tool was used to assess the risk of bias and methodological quality of the eligible studies. From the included studies, the rate ratio (RR) of pooled accuracy, sensitivity, specificity and their corresponding confidence intervals (CIs) were estimated for both PWI and PET.
Results: The systematic review and meta-analysis comprised 14 research studies, with a total of 542 patients. Although PET revealed a moderately higher accuracy and sensitivity when compared to PWI (RR: 0.94, 95% CI 0.86-1.02 and RR: 0.95 95% CI 0.85-1.06, respectively), the difference was not statistically significant (p>0.05). Similarly, while PWI demonstrated a moderately higher specificity when compared to PET (RR:1.10, 95% CI 0.98-1.23) but. However, no statistically significant difference between the 2 modalities was detected (p>0.05). Interestingly, we revealed that 18F-FET-PET was significantly more efficient than PWI in terms of accuracy (RR: 0.82, 95% CI 0.72-0.93) and sensitivity (RR: 0.72, 95% CI 0.62-0.83) (p>0.05).
Conclusion: Both PET and PWI yielded good diagnostic performance in distinguishing treatment-related changes from tumor recurrence, and neither modality seemed to be superior. PROSPERO ID: CRD42021288160
Brain tumors occur when abnormal and uncontrolled cell division appears in the brain. Nowadays, non-cancerous (benign) tumors and cancerous (malignant) tumors are the 2 groups of tumors recognized worldwide.1 Brain tumors can also be categorized as primary tumors, starting in the brain, and secondary tumors, also known as brain metastasis tumors, which most frequently have spread to the brain from primary tumors in other organs.2 The Central Brain Tumor Registry of the United States reported that primary brain tumors account for around 2% of all cancers, while metastases occur approximately in 10-20% of people with cancerous tumors and are 10 times more frequent than primary brain tumors.3 The annual frequency of primary brain tumors can reach 19 cases per 100,000 people. The incidence is 3 cases per 100,000 people at less than 4 years of age. The prevalence decreases between 15 and 24 years of age and then increases regularly to a peak of 19 cases per 100,000 people between 65 and 79 years of age.4
The World Health Organization (WHO) classifies brain tumors into different types, and each of them may be classified into diverse grades, including neuroepithelial and non-neuroepithelial tumors.5 The majority of primary tumors of the brain in adulthood are gliomas, with a prevalence of 45% among all brain tumors and 90% of primary brain tumors in elderly patients.6 Gliomas are classified into 4 grades (I, II, III, and IV) based on the WHO classification. Low-grade gliomas include grades I and II, while high-grade gliomas include grades III and IV. The grade IV glioma is also known as glioblastoma multiforme.7
Many studies have demonstrated a higher prevalence of brain cancer in developed countries. Furthermore, the prevalence of brain cancer depends on many factors, such as age, gender, and race. Meningioma and glioblastoma are mostly detected at approximately 65 years of age. However, the other types of brain tumors, such as pilocytic astrocytoma, germ cell, and pineal region tumors are detected at an early age.8 The prevalence of brain tumors is moderately greater in men as compared to that in women, but meningeal tumors seem to be 2 times as frequent among women. Regarding gliomas, their incidence is higher in men (7.7/100,000) as compared to that in women (5.61/100,000).9 The prevalence of primary brain tumors is higher in Asian, white, and black populations with regard to American and Indian Native race groups. Brain cancers have a 5-year survival rate of approximately 33%. In general, high performance status, young age, and low histopathological grade are positive prognostic characteristics for primary brain tumors.10
Therapeutic options are dependent upon the kind of brain tumor, as well as its location and size. Treatments for brain tumors include surgical resection of solitary lesions, stereotactic brain surgery and radiation with or without chemotherapy.11 Treatment-induced necrosis is a frequent treatment-related condition occurring during the treatment of gliomas, which is usually detected 3 to 12 months post-treatment.12 Indeed, after high radiation doses, patients usually develop constant or continuous enhancement detected with recurrent tumors (pseudoprogression), radionecrosis and inflammation. These conditions mimic tumor progression or recurrence after remission.13 Pseudoprogression appears as an increase in the size of the primary tumor or the appearance of a new lesion, thus resembling early progressive tumors.14 It is evident that misdiagnosis of recurrent brain tumors changes the treatment strategy, which leads potentially to unnecessary repeat surgery or non-effective second-line treatment.15 Histopathologic technique is the current gold standard to confirm the diagnosis of recurrent brain tumors, but biopsy is dangerous, with many adverse impacts (such as inflammation and hemorrhage).16 Hence, differentiating tumor recurrence from other types of treatment-related changes is challenging. Consequently, valid, effective and non-invasive imaging techniques are required to improve the post-treatment surveillance of patients.
Due to the disadvantages of histopathology, diverse neuroimaging techniques have been developed for the distinction of recurrent tumors from treatment-related changes. Magnetic resonance imaging (MRI) modalities, such as diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI), and nuclear modalities, including positron emission tomography (PET), which can be associated with computed tomography (PET-CT) or magnetic resonance imaging (PET-MRI), are usually used for this purpose.14
Nowadays, MRI is the standard neuroimaging modality for follow-up after treatment.15 Standard MRI is based on the magnetization properties of atomic nuclei. The most common MRI sequences are T1-weighted and T2-weighted scans.17 Even with the perfect ability to diagnosis brain tumors, the conventional MRI presents some limitations regarding the distinction between tumor recurrence and non-specific changes, which is especially relevant after therapy.
To surmount these deficiencies, PET based on radioactive amino acids and PWI are suggested as alternative modalities that may supply supplementary pathophysiological evidence to standard MRI. Perfusion-weighted imaging is one of the most broadly used clinical techniques. It is accurate, reliable, and safe, as well as radiation-free. There are 3 types of PWI-dynamic magnetic contrast-enhanced, dynamic contrast-enhanced, and arterial spin labeling.18 In recent years, PET using radioactive amino acids has been considered a very pertinent modality and is described as clinically helpful for managing patients with brain tumors.19 Different types of radioactively labeled tracers (such as, 11C-MET, 18F-FDG [fluorine18-fluorodeoxyglucose], 18F-FLT [fluorothymidine], or 18F-FET [fluorine-fluoro-ethyl-tyrosine]) are injected with PET to improve the detection of recurrent brain tumors.20 The first radiopharmaceutical tracer to be developed was 11C-methyl-L-methionine (MET), and 18F-FDG is the most broadly applied tracer.21 Nowadays, both of them are considered the best tracers for PET imaging of brain tumors.22 In summary, imaging findings from PWI and PET may contribute, in addition to MRI, to improve both diagnostic and therapeutic planning, particularly in clinically challenging situations. However, each of these modalities has its disadvantages. Standard MRI does not produce reliable data to distinguish radiation-related changes from true tumor progression, while Magnetic resonance (MR) spectroscopy and PET can provide false positive results regarding recurrent tumors.23 Despite their significant contribution to better differentiate recurrent tumors from treatment-related conditions, research comparing the effectiveness of PET and PWI is limited.
Therefore, this systematic review and meta-analysis was carried out to evaluate the diagnostic performance of PWI compared to PET for distinction between tumor recurrence and treatment-related changes. Then, we performed a subgroup analysis to investigate the effectiveness of each PET modality and tracer in comparison with PWI.
Methods
This study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.24 The writing followed the Meta-analysis of Observational Studies in Epidemiology scheme.25 The Embase, Web of Science, PubMed, the Cochrane library, and CINAHL databases were used to search potentially interesting articles published from database inception until August 2021. A systematic search was performed by 2 independent reviewers, using the following terms “perfusion MRI” or “perfusion magnetic resonance imaging” or “perfusion-weighted imaging” or “PWI” and “positron emission tomography” or “PET” and “glioma” or “glioblastoma” or “brain tumor” and “recurrent” or “recurrence.”
Relevant articles were screened by title and abstract after suppression of duplicates. Articles were included if they described the use of PWI and PET to detect brain tumor recurrence. Then, they were read in full text to verify eligibility.
The inclusion criteria were as follows: i) patients with suspected radiological recurrence at the site of previously irradiated brain tumor; ii) histopathology or clinicoradiological diagnosis used as a reference to differentiate treatment-related effects from recurrent tumors; iii) use of PWI and PET; iv) publications reporting sufficient information on the sensitivity and specificity of each test; and v) studies published as original articles. The exclusion criteria were as follows: i) no full text electronically available; ii) publication in a language other than English; iii) comments, letters, editorials, protocols, guidelines, and review papers; iv) use of other imaging techniques (DWI or conventional MRI); or v) studies with insufficient outcome data.
Two independent reviewers independently retrieved information from the eligible articles following the inclusion and exclusion criteria, and the information was collected on a standardized data sheet that included author name, year, type of study, country, number and age of participants, diagnosis of tumor, time since radiation completed (days), modality and optimal cutoff, type of PET, the follow-up period and the reference technique used to differentiate pseudoprogression from recurrent tumors. The methodologic quality of the included studies was evaluated independently by 2 authors using the Quality Assessment of Diagnostic Accuracy Studies-2 tool, which includes four criteria—”patient selection,” “index test,” “reference standard,” and “flow and timing”.26 The outcomes of interest were the accuracy, sensitivity, and specificity of PET and PWI to detect tumor recurrence.
Statistical analysis
In this meta-analysis, accuracy, sensitivity and specificity measures of the best effective parameter were pooled from each article. The statistical analyses were carried out using RevMan Version 5.4 (Cochrane Collaboration, Oxford, United Kingdom). Rate ratios (RRs) with 95% confidence intervals (CIs) were adopted to compare the sensitivity, specificity and accuracy between PWI and PET. A p value of <0.05 was considered as the level of significance. The Cochrane Chi-squared test was conducted to assess heterogeneity among studies, with p<0.05 indicating the existence of heterogeneity. The I2 value was used to estimate the impact of heterogeneity on the meta-analysis; I2≥50% and p<0.05 indicated a moderate to high degree of heterogeneity among pooled studies. When I2<50% and p>0.05, a fixed effects model was conducted; if not, a random effects design was adopted.27 Additionally, we carried out subgroup and sensitivity analyses to evaluate the likely cause of heterogeneity. Egger’s test was carried out via Statistical Package for Social Sciences version 25 (IBMCorp, Armonk, NY, USA) to evaluate publication bias. This latter was further assessed based on the visual inspection of the symmetry in funnel plots.
Results
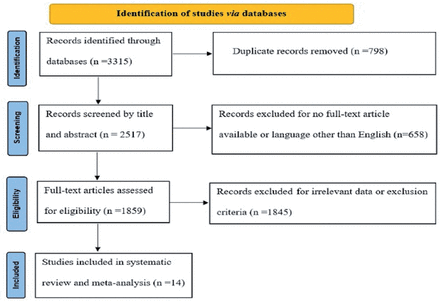
Database searches identified 3315 articles to be screened, of which 1859 studies were revealed as potentially eligible and retrieved for full text review. Eligibility criteria were met by 14 studies, which were included in this systematic review and meta-analysis. The PRISMA study flowchart is shown in Figure 1.
- An outline of the PRISMA guidelines used to conduct this meta-analysis.
The 14 included studies were published between 2008 and 2021 and distributed among 10 countries.28-41 Among the 14 articles included in this meta-analysis, 3 were prospective studies and 11 were retrospective studies. The sample size of the included articles varied from 10 to 104 patients and the age varied between 8 and 81 years. Histopathology was the reference standard to detect tumor recurrence. The following 4 types of amino acid tracers were used with PET: 18F-FDG (7 articles), 11C-methionine (5 articles), 18F-FET (2 articles), and fluorodopa (one article). The features of the studies are recapitulated in Table 1.
- Features of included articles (N=14).
- Features of included articles (N=14) (continuation).
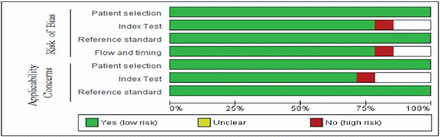
Regarding patient selection, enrollment was cited in all articles. Considering the index test criteria, a specified threshold was mentioned in the majority of the articles. In addition, a low risk of bias was revealed in many articles in terms of flow and timing criteria (75%). Moreover, all studies had a low risk in terms of reference standard, since the diagnosis was based on histopathology. A recapitulation of the risk of bias is presented in Figure 2.
- Risk of bias items presented as percentages across all articles. PET: positron emission tomography, PWI: perfusion-weighted imaging, CI: confidence interval
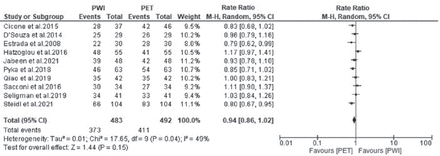
Of the 14 included studies, 10 studies reported the accuracy of PWI and PET. The heterogeneity (χ2=17.65, p=0.04, I2=49%) was high, so a random effects model was adopted. The forest plot showed that the accuracy outcome was not significantly different between PWI and PET (RR=0.94, 95% CI: 0.86-1.02; p=0.15) (Figure 3).
- Forest plot of the rate ratio of accuracy between perfusion-weighted imaging (PWI) and positron emission tomography (PET). CI: confidence interval
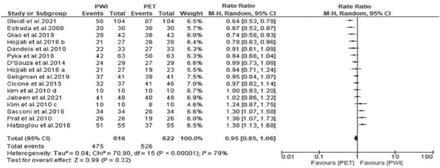
All the studies reported the sensitivities of both PWI and PET. A random effects model was adopted because the heterogeneity was high (χ2=70.90, p<0.00001, I2=79%). The forest plot demonstrated that the sensitivity outcome was not significantly different between PWI and PET (RR=0.95, 95% CI: 0.85-1.06; p=0.32) (Figure 4).
- Forest plot of the rate ratio of sensitivity between perfusion-weighted imaging (PWI) and positron emission tomography (PET). CI: confidence interval
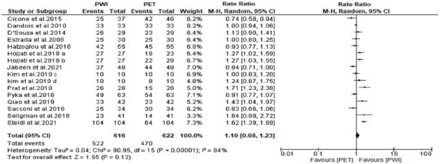
All the studies reported the specificities of both PWI and PET. A random effects model was adopted as the heterogeneity was high (χ2=90.95, p<0.00001, I2=84%). The forest plot revealed that the specificity outcome was not significantly different between PWI and PET (RR=1.10, 95% CI: 0.98-1.23; p=0.12) (Figure 5).
- Forest plot of the rate ratio of sensitivity between perfusion-weighted imaging (PWI) and positron emission tomography (PET). CI: confidence interval
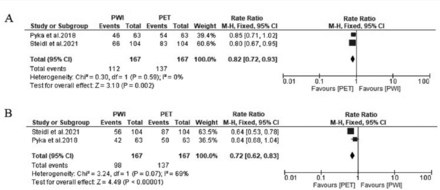
Exploratory subgroup analysis suggested that an only prospective study design was a cause of heterogeneity for the accuracy outcome (p=0.03). Regarding sensitivity, all parameters contributed to the heterogeneity, except the 18F-FET and 11C-methionine tracers (p=0.07). Similarly, all parameters caused heterogeneity for the specificity outcome, except the prospective study design (p=0.44) (Table 2). Additionally, we noticed that the 3 outcomes were not significantly different between PWI versus (vs) PET, PWI vs PET/CT, and PWI vs PET/MRI (p>0.05 for all). Interestingly, we revealed a significant difference in terms of accuracy and sensitivity when using the 18F-FET tracer (p<0.05). Indeed, 18F-FET-PET proved to be more efficient than PWI in differentiating recurrent tumors from treatment-related changes (Figure 6).
- Forest plot showing the rate ratio of (A) accuracy and (B) sensitivity between PWI and positron emission tomography (PET).
A leave-one-out sensitivity analysis was conducted to detect the likely cause of heterogeneity in the pooled RR of accuracy, sensitivity, and specificity between PWI and PET. The findings revealed that the outcomes did not differ noticeably, which indicates that this meta-analysis had solid precision. Indeed, the RRs of accuracy, sensitivity, and specificity ranged from 0.93 (95% CI 0.79-1.01) to 0.98 (95% CI 0.89-1.10), from 0.93 (95% CI 0.80-1.02) to 1.04 (95% CI 0.93-1.17), and from 0.97 (95% CI 0.88-1.17) to 1.13 (95% CI 1.03-1.31), respectively.
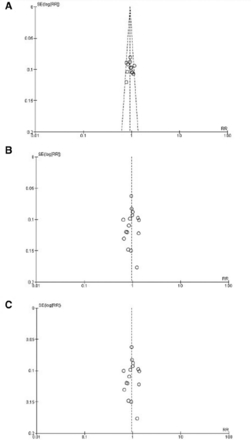
We demonstrated no proof of publication bias for all 3 outcomes analyzed by using Egger’s regression test (p>0.05). Moreover, visual inspection of the funnel plot revealed symmetry (Figure 7).
- Funnel plot showing no publication bias in terms of (A) accuracy, (B) sensitivity, and (C) specificity among the studies.
Discussion
The present systematic review and meta-analysis comprising 14 articles is, to the best of our knowledge, the first to assess the relative effectiveness of PWI and PET in the distinction between early recurrent tumors and changes related to treatment. Discriminating between treatment change and tumor recurrence has been a subject of expanded medical interest using diverse neuroimaging techniques.
Consistent with previous research articles on the distinction between true tumor recurrence and treatment-related conditions, this systematic review and meta-analysis included articles of patients with suspected radiological progression at the site of previously irradiated brain tumors, at an interval not only <3 months but also >3 months after radiation therapy. In fact, treatment-related conditions are a wide class that consists of diverse well-defined clinical units regrouping pseudoprogression, as well as radiation necrosis and mixed response.42 Pseudoprogression usually appears weeks to 3 months after the achievement of treatment, while radiation necrosis occurs in most cases 18-24 months to years afterward.43 Hence, distinction of recurrent tumors from treatment-related alterations is challenging, since both disorders presented similar neurological signs.44 Consequently, the prompt detection of recurrent tumors (within 3 months) allows physicians to choose between changes in chemotherapy and repeat surgery in order to ameliorate the patient’s course. Standard MRI techniques have low efficiency, as revealed by Taghipour Zahir et al,45 who revealed that MRI yielded a limited specificity of 25%.45 Many studies have discussed the limitations of conventional imaging techniques in differentiating tumor progression/recurrence and immunotherapy responses in order to highlight the need for developing advanced imaging methods to overcome these limitations.46,47
Consequently, PWI, and PET have been used for their important role in differentiating recurrent tumors from treatment-related changes. Diverse research has evaluated the effect of amide proton transfer-weighted MRI or MR spectroscopy, investigating particular imaging parameters as important predictors of tumor recurrence.
In the present meta-analysis including 14 studies, PET and PWI were found to have the same effectiveness in distinguishing true tumor progression from treatment-related changes after radiation treatment, regardless of the modality or amino acid tracer used with PET. Positron emission tomography and PWI are promising techniques, but comparative studies on their effectiveness have shown heterogenous results in terms of early progression. Steidl et al41 reported that PET could differentiate glioma progression from treatment-related changes with a specificity of just 62% against 100% for PWI.41 Similarly, Seligman et al41 revealed that the best-performing PET and PWI cutoffs achieved a specificity of 56% and 89%, respectively, indicating that PWI is more efficient than PET in detecting recurrent tumors.40 In this context, Patel et al48 demonstrated that PWI-derived thresholds differentiating true tumor progression from other conditions revealed high accuracy. Obviously, additional standardization and investigation are needed before adopting any specific PWI strategy, due to the significant variability in optimal reported thresholds.48
On the other hand, some studies showed that PET was more efficient than PWI. For example, Cicone et al28 revealed that PET was a highly accurate tool for differentiating brain radionecrosis from recurrent brain metastases after stereotactic radiosurgery, with a sensitivity of 93.3% and a specificity of 90.9%, which is higher than those yielded by PWI (86.7% and 68.2%, respectively).28 Similar findings have been highlighted by Sacconi et al39 who demonstrated that PET seems to outperform PWI in terms of specificity (89.7% vs 74%).
Regarding the amino acid tracers used with PET, we have noticed that 18F-FET-PET was more efficient than PWI in terms of accuracy and sensitivity. A previous meta-analysis demonstrated that 18F-FDG and 11C-MET PET seemed to possess a relatively high accuracy for detecting the recurrence of gliomas.49 Yet, studies investigating 18F-FET and 18F-FLT cited good sensitivity, especially for high-grade tumors.50,51,18F-FET and 18F-FLT are newly discovered PET tracers. Over the past years, they have been investigated in clinical use only. Only some studies have evaluated the diagnostic accuracy of these tracers. Although pertinent findings have been cited, supplementary validations are required. Recently, de Zwart et al52 carried out a meta-analysis to assess the diagnostic performance of 11 PET tracers for the distinction of tumor recurrence from radiation-related alteration in high-grade tumors. The findings obtained showed that 18F-FET and 11C-MET had a higher sensitivity than 18F-FDG in the distinction of recurrent tumors from radiation-related alterations. However, the evidence for the remaining tracers was limited.
The major advantage of PWI in comparison with PET in brain tumors include whole brain coverage and simpler post-processing methodology.53 In addition, the software used to postprocess data yielded by PWI is broadly accessible and quite easy to use. Perfusion modalities present to the user the capacity to investigate the brain’s microvasculature from a different perspective. This may provide a real evaluation of tumor angiogenesis. Furthermore, perfusion MRI is a very safe imaging method, unlike PET which presents a risk of radiation exposure.54 The main findings were that costs were significantly lower for PWI compared with PET in the evaluation of brain tumors. Perfusion-weighted imaging was economically attractive, especially because of fewer invasive processes, whereas PET was considered an expensive imaging test with a higher price and no notable difference in terms of sensitivity and specificity compared with PWI. 55,56 Consequently, PWI as a non-invasive, convenient and less expensive modality, seems to be the preferred imaging strategy in post-treatment surveillance of brain tumors.
Study limitation
There are limited number of studies that compare other tracers or PET vs different neuroimaging techniques (such as, PWI). Thereby, research articles conducting head-to-head comparisons between PET tracers and alternative MR imaging techniques (such as, MR spectroscopy, DWI, and PWI or between different PET tracers) are particularly needed.
In the present study, we carried out literature searches in 5 different databases. The principal strong items of this article are the large scale of studies and considerable number of participants analyzed. Although unpublished articles were not included in our study, the funnel plot did not show a publication bias. Additionally, the sensitivity analyses showed that the estimated RR of accuracy, sensitivity and specificity outcomes were reliable and not effected when a single study was omitted.
Study limitation
This meta-analysis relates to the elevated rate of heterogeneity between the articles. Regarding the subgroup analysis, heterogeneity could be likely due to differences in study design, PET modalities, and amino acid tracer used. These differences caused major difficulties in synthesizing all available studies. Hence, considerable heterogeneity, which is expected in meta-analysis studies, can alter the interpretability of results.57 Consequently, the findings of the present work have to be analyzed with attentiveness.
In conclusion, PET and PWI show comparable accuracy, specificity and sensitivity in differentiating recurrent tumors from radiation-related alterations. Taking into account the advantages of PWI compared to PET in terms of safety, availability and cost, PWI seems to be more favorable than PET for the follow-up of patients.
These techniques presented conflicting results between some studies. Provided that the neuroimaging distinction between recurrent tumors and treatment-related changes represents a real challenge and has an impact on the choice of therapeutic strategy, supplementary works with larger sample sizes are required to produce more significant findings.
Acknowledgment
The authors would like to thank Proof-Reading-Service (www.proof-reading-service.com) for the English language editing. The authors would also like to acknowledge all the associated personnel in any reference that contributed in/for the purpose of this research.
Footnotes
Disclosure. Author have no conflict of interests, and the work was not supported or funded by any drug company.
- Received December 30, 2021.
- Accepted June 1, 2022.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.