Abstract
Spontaneous cerebrospinal fluid (CSF) fistula is a rare entity, most commonly occurs at the ethmoid roof, cribriform plate, or the sphenoid sinus; at the perisella, inferolateral or pterygoid recesses. Imaging plays a major role in diagnosis, thereby guiding the treatment of a spontaneous CSF fistula, evolving multiple modalities. We report a case of a patient with spontaneous Meningio-encephalocele presented as an expansile lytic lesion in the left pterygoid body, this patient was successfully treated surgically.
A spontaneous cerebrospinal fluid (CSF) fistula is a rare disorder constitutes of nasal discharge of CSF with no underlying relation to trauma, surgery, malformation, tumor or a previous radiation therapy. Spontaneous CSF fistulas have a common incidence in middle-aged women and in patients with raised intracranial pressure. The usual clinical presentation is otorrhea, rhinorrhea, headache, seizure, meningitis, or as an incidental finding. The most common locations in the skull base are at the ethmoid roof, cribriform plate, the sphenoid sinus; at the perisella, inferolateral or pterygoid recesses. Imaging plays a major role in diagnosis, thereby guiding the treatment of the spontaneous CSF fistula. Imaging involves a multidetector, thin-section computed tomography (CT) imaging; utilizing bone algorithm, CT cisternography, conventional Magnetic Resonance Imaging (MRI) and Magnetic Resonance (MR) cisternography.
We report a case of a patient with spontaneous meningoencephalocele that presented initially as an expansile lytic lesion in the left pterygoid body, the skull base bony defect in the left middle cranial fossa consisted of cortical permeation and multiple pitting on its inner cortex. It was successfully managed by endoscopic skull base reduction of the left pterygoid body meningoencephalocele and fixation of the bone defect by application of a multilayer nasoseptal flap, dural and fat patches.
Case Report
Patient information
A 29-year-old female patient, known to have epilepsy was referred to Neuroradiology for a seizure workup. The seizure had been controlled by 200 mg carbamazepine (Tegretol) twice a day (the treatment level is 31.5). Additionally, the patient was on a 20 mg vitamin K oral supplement once a day. Her last seizure attack had been since 5 years and was a tonic-clonic episode. She recalled having a remote history of head trauma at the age of 5, that did not require any kind of medical imaging or intervention. The patient declined any past history of prior skull base fracture or meningitis. The patient had never complained of nasal or ear discharge, headache, or any visual symptoms.
Clinical findings
A baseline electroencephalogram (EEG) and neurological examination were normal. The body mass index (BMI) was 27 (weight: 64 kg, height: 154 cm), and the vital signs were stable. The nasal scope was clear with patent nasal cavity. No polyposis or mases were seen.
Diagnostic assessment
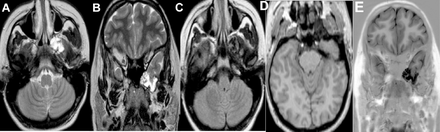
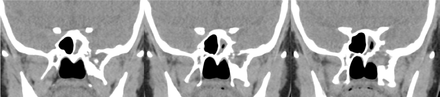
Upon reporting an MRI brain requested for seizure disorder, there was an incidental finding of a lobulated T1-weighted images /fluid-attenuated inversion recovery (FLAIR) hypointense and T2-weighted images hyperintense lesion in the left middle cranial fossa, in continuation with the anterior aspect of the left temporal pole, descending to the left pterygoid process through a very thin CSF longitudinal column. Intralesional signal voids were noted, likely due to a turbulent flow (Figure 1). Further multidetector, thin-section CT scan with bone algorithm was performed, showed an expansile lytic lesion in the left pterygoid body, medial and lateral pterygoid plates, the lesion was extending to the left sphenoid body through a bone defect, consisted of cortical permeation and multiple pitting on its inner cortex (Figure 2). No signs of increased intracranial pressure were noted in the performed cross-sectional studies, such as an empty or partially empty sella, vertical tortuosity of the optic nerve or prominence of the perioptic CSF spaces. Orbits were unremarkable, with no flattening of the posterior sclera. Computed tomography and MR cisternography were not performed, neither intrathecal administration of contrast, as the patient was referred initially for epilepsy work-up that required a different imaging protocol that’s when the patient was found to have an incidental expansible lytic bony lesion in the left pterygoid process.
Axial and coronal T2 (A, B), axial FLAIR (C) and axial and coronal T1 SPGR (D, E). A lobulated hypointense T1/ FLAIR and hyperintense T2 left middle cranial fossa lesion was observed in continuity to the left anterior temporal pole, descending to the left sphenoid and the left pterygoid process through a thin CSF column. T1 SPGR showed intralesional signal voids due to turbulent flow.
Axial (A), sagittal (B) and coronal (C) multidetector, thin-section bone algorithm CT images. An expansile lytic lesion was observed in the left sphenoid body, extending to the pterygoid body and the medial and the lateral pterygoid plates. The bony defect seen in the left sphenoid body consisted of cortical permeation and multiple pitting on its inner table.
Therapeutic intervention
Endoscopic transpterygoid reduction of the meningoencephalocele, skull base reconstruction and repair were performed along with fixation of the bone defect by a left nasoseptal flap (multilayer repair) and a (synthetic dural) and fat patches. No active CSF leak was detected intraoperatively, despite the use of intrathecal fluorescein. Unfortunately, intraoperative photographic documentation was not available.
Follow-up and outcomes
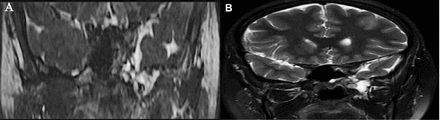
Surgery went uneventfully with no residual or recurrence of the meningoencephalocele. The patient presented to the clinic in the immediate first and second months postoperatively and also over the period of 20 months. The patient’s clinical assessment was satisfactory in these visits. A postoperative CT sinuses was performed, demonstrated a successful reduction of the meninigo-encephalocele with a visible fat patch. Diffuse mucosal thickening and hyperdense mucosal opacification was seen in the left maxillary sinus, representing changes of chronic sinusitis (Figure 3). Postoperative MRI was also carried out, again demonstrated the surgically reduced meninigo-encephalocele; that was seen confined within the boundary of the left middle cranial fossa. An underlying thin T2 hypointense line was seen, representing the dural patch, more conspicuous in the coronal T2 and steady state free precession (SSFP) images (Figure 4).
A post-operative non-enhanced coronal CT images at the level of middle cranial fossa. A soft tissue at the skull base is seen representing multilayer flap with underneath fat patch. Inflammatory changes within the left maxillary sinus is also seen.
A postoperative coronal SSFP (A) and T2 (B). Successful reduction of the left middle cranial fossa meningo- encephalocele is shown. An underlying thin dark layer is demonstrated representing the dural patch.
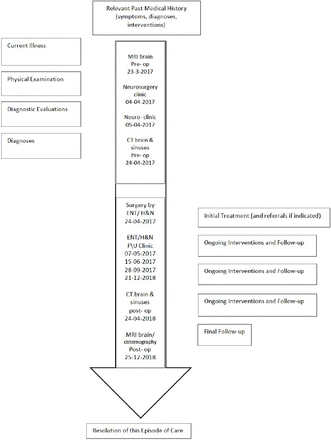
A timeline table summarizing the chronological sequence of the case report; including the clinical presentation, diagnosis, management and follow up is displayed in (Figure 5).
Timeline table summarizing the chronological sequence of the case report.
Discussion
Our patient was diagnosed incidentally during a seizure workup, with no specific clinical symptoms suggestive of active CSF leak. A spontaneous, or primary CSF fistula is considered as a separate entity in patients with no other discernible etiology for their CSF leak; i.e. history of trauma, tumor, or congenital abnormality.1 A spontaneous CSF leak is most commonly found in obese, middle-aged women, and has also been linked to a rare manifestation of idiopathic intracranial hypertension.1-8 A etiopathogenesis that combines physiologic and anatomic factors is readily favored based on CT and MR imaging observations of associations with pneumatization of the inferior lateral recess of the sphenoid sinus, persistent Sternberg’s canal, arachnoid pits, and an empty or partially empty sella.1,3-9
One theory states that spontaneous CSF leaks occurs due to chronic raised intracranial pressure resulting in arachnoid granulations that fill small pits in the inner table of either the calvarium or the sinus wall. The dura becomes thin, and the small diverticula of the arachnoid extend through the defects and rupture.
Impairment of the CSF absorption with a subsequent transient elevation in pulsatile CSF pressure is another theory that can lead to dural herniation and CSF leak over the weakened anatomical sites in the skull base.5
A spontaneous CSF fistula common sites of occurrence in the skull base are the ethmoid roof, cribriform plate, the sphenoid sinus; at the perisella, inferolateral or pterygoid recesses.5 Cerebrospinal fluid fistulas have a predilection at the inherent weakened areas; adjacent to the pneumatized paranasal sinuses, i.e. the sphenoid sinus, the floor of the middle cranial fossa, the tegmen tympani, the roof of the Eustachian tube, the jugular foramen and between the sigmoid sinus and the bony labyrinth. The cribriform plate and the fascia of the sellar diaphragm are also commonly involved.10
In a retrospective study of 26 patients, the clinical presentations constituted of CSF leaks in 12 patients (46%), headaches in 7 patients (27%), and seizures in 7 patients (27%). Some of the patients also presented with meningitis, cranial neuropathy, nasal fullness, and proptosis. A lateral sphenoid sinus CSF leak was a solitary incidental finding that’s been identified in a single patient.9 Risk for ascending infection and meningitis is predisposed in untreated cases.1
Imaging is a key factor in diagnosing spontaneous CSF leaks. Utilizing thin-section, multidetector CT scans, small defects can be visualized with a reported sensitivity as high as 92% and a specificity of 100%. The CT findings include skull base bone defects, paranasal sinuses opacification with/without air-fluid level. Magnetic resonance imaging is ultimately performed upon identification of bone defects with an accompanied sinus lobular or nondependent opacification, usually indicating an underlying association with a meningocele or encephalocele. Magnetic resonance cisternography typically involves heavily T2-weighted, fast spin echo sequences, with fat suppression and subtraction of the adjacent background tissue signal, in order to enhance imaging of the fistulous tract, or the CSF column.1
In conclusion, this case report implies the importance of diagnosing subtle CSF fistulas with/without meningio-encephalocele especially when it presents incidentally, utilizing cross sectional imaging and applying the cisternography techniques whenever possible.
Acknowledgements
The authors gratefully acknowledge the professional manuscript services of American Journal Experts.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received September 13, 2018.
- Accepted January 30, 2019.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.