Abstract
Benign fibrous histiocytoma (BFH) within the intracerebral region is remarkably rare. Our report details 2 cases of unusual BFH instances that exhibit no adhesion to the dura mater or cerebral falx, accompanied by a comprehensive literature review. While magnetic resonance imaging demonstrates specific characteristics for BFH, it does not readily differentiate BFH from more common brain neoplasms like gliomas and metastatic tumors. The definitive diagnosis of BFH depends primarily on histopathological and immunohistochemical examinations. Total surgical resection is considered an efficacious therapeutic approach, emphasizing the necessity for prolonged postoperative surveillance to detect any potential tumor recurrence or metastasis.
Benign fibrous histiocytoma (BFH) is a seldom-encountered mesenchymal tumor predominantly found in the dermal and soft tissue layers, with occasional manifestation within bones and retroperitoneal structures.1 Intracranial presentations of BFH are exceptionally rare, with fewer than 20 documented cases available in the literature. These incidents predominantly involve extra-axial tumors originating from the scalp, cranial bone, or dura mater, with subsequent infiltration into the cerebral tissue.1-5 Specifically, intracerebral BFH cases are exceptionally uncommon, with only 4 documented known cases to date.1-3 Notably, within the category of intra-axial brain tumors, those not associated with the dura mater or cerebral falx are particularly rare and frequently misidentified as meningiomas prior to surgical intervention.3 This report aimed to introduce 2 cases of intra-axial BFH, both conclusively confirmed through surgical and histopathological assessments, distinguished by their spatial disassociation from the meningeal layers.
Case Report
Case 1. Patient information
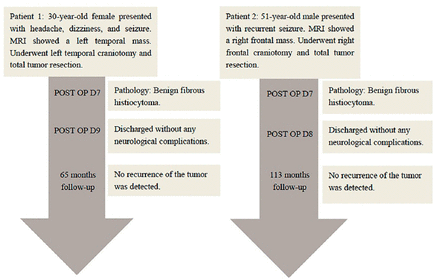
A female patient, aged 30, was admitted to our hospital (Huashan Hospital North, Shanghai, China) due to a 3-month history of persistent headaches and dizziness, accompanied by a generalized seizure that occurred one week prior to her admission (Figure 1).
- Timeline of clinical course and outcome for both BFH patients. BFH - benign fibrous histiocytoma
Clinical findings
Upon physical examination, no abnormal signs or symptoms were detected.
Diagnostic assessment
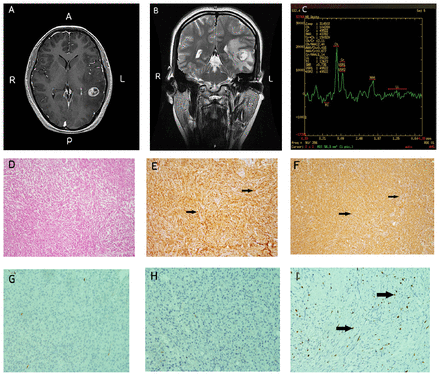
Preoperative magnetic resonance imaging (MRI) of the brain revealed a well-defined mass, measuring 1.8×1.5 cm, situated in the left temporal lobe. The lesion displayed mixed iso-hypointensity on T1-weighted images, iso-hyperintensity on T2-weighted images, and iso-hypointensity on diffusion-weighted imaging. Upon contrast-enhancement, heterogeneous enhancement of the lesion was observed in T1-weighted images. Magnetic resonance spectroscopy (MRS) indicated an increased choline-to-N-acetylaspartate (CHO/NAA) ratio of 2.4. Notably, the T2-weighted MRI images displayed significant peritumoral brain edema. Axial and coronal MR images confirmed the absence of contact between the tumor and the cerebral falx or dura mater (Figure 2A-C). Chest computed tomography (CT) scan showed no apparent abnormalities, and her laboratory results were within normal limits.
- Case 1 - Preoperative Imaging and Histopathological Analysis. (A) Gadolinium-enhanced MRI revealed a lesion in the left temporal region exhibiting heterogeneous enhancement. (B) Coronal T2-weighted MRI displayed no adherence to the dura mater. (C) MRS indicated maximal CHO/NAA ratios of 2.4. Panels (D) through (I) present IHC examination at 100× original magnification: (D) Hematoxylin and eosin (H&E) staining. (E) CD68 immunopositivity (positive cells indicated by arrows). (F) Vimentin immunopositivity (positive cells indicated by arrows). (G) Negative glial fibrillary acidic protein (GFAP) staining. (H) Negative epithelial membrane antigen (EMA) staining. (I) Weakly positive Ki-67 staining (positive cells indicated by arrows).
Therapeutic intervention
A left temporal craniotomy was performed under general anesthesia for tumor excision. Intraoperatively, a grey-red tumor with a slightly soft consistency was identified, situated approximately 2 cm beneath the cortex as guided by MRI navigation. The tumor displayed an unclear boundary with adjacent healthy brain tissue. The tumor exhibited substantial vascularity with a rich blood supply, but it did not adhere to the dura mater or cerebral falx. A complete resection of the tumor was achieved. Her postoperative period was uneventful, and she recovered well without any ensuing neurological deficits.
Postoperative histopathological evaluation demonstrated that the lesion primarily consisted of foamy cells arrayed in sheets within a mildly fibrous stroma. While mild nuclear atypia was observed, no mitotic activity was identified. Peripheral regions of the lesion exhibited small aggregates of benign lymphocytes. Immunohistochemical (IHC) analysis demonstrated positive staining for the cluster of differentiation 68 (CD68), vimentin (Vim), integrase interactor 1 (INI-1), Nestin, smooth muscle actin (SMA), and CD163. Partial expression was observed for S100 protein, P53, leukocyte common antigen (LCA), and desmin (DES). In contrast, markers such as glial fibrillary acidic protein (GFAP), epithelial membrane antigen (EMA), progesterone receptor (PR), cytokeratin (CK), estrogen receptor (ER), CK5/6, thyroid transcription factor-1 (TTF-1), P63, P40, human melanoma black 45 (HMB45), SRY-Box transcription factor 10 (SOX-10), actin, myogenin, and MyoD1 did not exhibit staining. The Ki-67 labeling index was found to be less than 10% in the majority of stained regions (Figure 2D-F). These findings consolidated the diagnosis of BFH.
Follow-up and outcomes
Her recovery progressed well post-surgery, and no recurrence of the tumor was detected at the 65-month follow-up.
Case 2. Patient information
A male patient, aged 51, underwent evaluation due to a one-year history of recurrent epileptic seizures (Figure 1).
Clinical finding
Upon neurological examination, no abnormalities were detected.
Diagnostic assessment
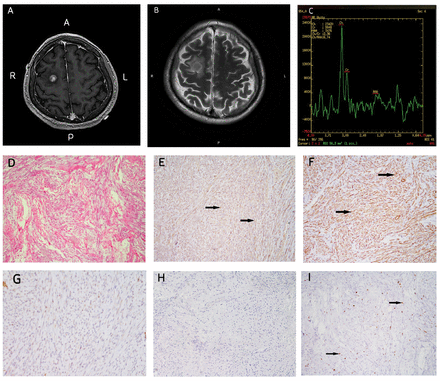
A preoperative brain MRI disclosed a well-defined mass, measuring 1×1 cm, located within the right frontal lobe. This mass exhibited heterogeneous enhancement on the T1-weighted contrast image, while MRS indicated an elevated CHO/NAA ratio of 6.74, indicative of high metabolic activity (Figure 3A-C). Notably, pronounced peritumoral brain edema was evident on T2-weighted MR images. Routine biochemical and hematological tests, and a chest CT scan, showed no abnormalities.
- Case 2 - Preoperative imaging and histopathological analysis. A) Gadolinium-enhanced MRI revealed a lesion in the right frontal region with heterogeneous enhancement. B) Axial T2-weighted MRI displayed peritumoral edema. C) MRS indicated maximal CHO/NAA ratios of 6.74. Panels (D) through (I) present IHC examination at 100× original magnification: D) H&E staining. E) Vimentin immunopositivity (positive cells indicated by arrows). F) Smooth muscle actin (SMA) immunopositivity (positive cells indicated by arrows). G) Negative glial fibrillary acidic protein (GFAP) staining. H) Negative epithelial membrane antigen (EMA) staining. I) Weakly positive Ki-67 staining (positive cells indicated by arrows).
Therapeutic intervention
He underwent a right frontal craniotomy for a complete tumor resection. Intraoperatively, the tumor was located 1 cm beneath the cortex, displaying a grey-red, tenacious texture, with no adherence to the dura mater. His postoperative recovery was uneventful, with no subsequent neurological impairments.
Microscopic evaluation of the excised tumor revealed numerous spindle-shaped cells organized in a storiform pattern. The nuclei of these tumor cells displayed mild irregularity and atypia. Immunohistochemical (IHC) analysis yielded positive staining for Vim, P53, and SMA. Conversely, negative staining was observed for GFAP, EMA, S100 protein, CD34, PR, CK7, TTF-1, Synapsin, Oligodendrocyte transcription factor 2, Isocitrate dehydrogenase 1, CD68, DES, and LCA. The Ki-67 labeling index was found to be less than 10% in the majority of stained regions (Figure 3D-F). These findings supported the definitive pathological diagnosis of BFH.
Follow-up and outcomes
Following the surgery, he experienced a successful recovery and resumed his professional activities. He remained healthy at the 113-month follow-up, showing no evidence of tumor recurrence.
Discussion
Intracranial BFH is a remarkably rare neoplasm, with less than 20 reported cases, in contrast to approximately 40 cases of the more common intracranial malignant fibrous histiocytoma (MFH).1-7 These 2 pathologies exhibit distinct demographic profiles. Intracranial MFH predominantly affects individuals at an average age of 38.7 years, with a higher incidence in males, and the male-to-female ratio is about 2:1.6 Conversely, intracranial BFH is more commonly diagnosed in females, and the female-to-male ratio is 3:1, and tends to manifest in younger individuals, with an average age of 25.6 years, ranging from as young as 11 months to 56 years.1-5 The significant demographic variations between these conditions warrant further exploration to elucidate potential underlying factors contributing to such differences.
The etiology of MFH is multifactorial and encompasses factors such as prior irradiation, surgical interventions, trauma, bone infarctions, Paget’s disease, and fibrous dysplasia.5 In sharp contrast, the causal factors for BFH remain elusive, although there are occasional suggestions of an association with past cranial trauma.5 Elucidating the precise etiology of BFH represents a direction for future research endeavors.
The histogenesis of intracranial fibrous histiocytomas (FHs) remains debated among researchers. Some hypotheses suggest that FHs connected to the dura mater may originate from mesenchymal stem cells, whereas exclusively intracerebral FHs may stem from cells within the perivascular pial sheath.2 Remarkably, in both cases presented in this report, BFHs were situated entirely within the brain parenchyma, suggesting a potential origin in the perivascular pial sheath.
The BFH typically exhibits non-specific imaging characteristics. On T1-weighted MRI, the lesions often depict hypo- or isointensity, while their appearance on T2-weighted images may vary from hypo- to hyperintensity. Post-contrast T1-weighted images may display patterns of enhancement that are either heterogeneous or homogeneous.3,5,8-10 In the presented cases, MRI revealed relatively well-defined intra-axial masses with heterogeneous enhancement and significant peritumoral edema. MRS results demonstrated maximal CHO/NAA ratios of 2.4 and 6.74, respectively, the differential diagnosis from inflammatory lesions. However, it is critical to conduct further investigations to elucidate the relevance of distinguishing BFH from MFH. So intracranial extra-axial BFH should be differentiated from meningioma, solitary fibrous tumor and osteoma, intra-axial BFH should be differentiated from malignant glioma, metastatic tumor, lymphoma, cavernous hemangioma and inflammatory lesions. In this case, both tumors were located in the brain and were initially misdiagnosed as gliomas before operation, likely due to the extremely low incidence of intracranial BFH. Therefore, the tumors exhibiting homogeneous or heterogeneous enhancement on MRI, relatively clear boundaries, peripheral edema, and a high CHO/NAA ratio on MRS should be considered the rare intracranial BFH as a potential differential diagnosis.3,5,8-10
The diagnosis of BFH predominantly relies on histopathological evaluation and immunohistochemical (IHC) analysis. Histologically, BFH is marked by the proliferation of spindle-shaped fibrohistiocytic cells, with occasional occurrences of multinucleated giant cells and foam cells, typically organized in a storiform or swirling pattern. Importantly, malignant attributes such as nuclear atypia, pleomorphism, atypical mitosis, and necrosis are rarely found.5 The IHC represents a valuable tool for distinguishing BFH from other tumor types. For instance, GFAP is generally absent, facilitating the exclusion of pleomorphic xanthoastrocytoma. Conversely, EMA assists in differentiating BFH from meningiomas, although approximately 20% of BFH cases may exhibit EMA positivity.1 Ki-67 expression aids in discerning benign from malignant forms of FH.3 In certain instances, electron microscopy may provide additional diagnostic insights.1
The primary treatment modality for intracranial BFH centers around complete surgical resection, typically resulting in a favorable prognosis. However, it is essential to recognize that certain tumors may recur or malignantly transform, particularly those originating from the dura mater. Several cases have been documented in the literature, including instances of an intracranial BFH emerging from the pterygopalatine fossa recurring after near-total resection, a tumor in the occipital/suboccipital region that recurred and metastasized post-subtotal resection, and an initially benign intraventricular tumor that transitioned into MFH five years post-surgery.1,4 Consequently, close postoperative monitoring with regular imaging and biopsy is highly recommended for intracranial BFH patients. Radiotherapy plays a limited benefit in the treatment of FH due to its unsatisfactory outcomes. The potential efficacy of chemotherapy in intracranial FH remains a subject of investigation, although promising results have been observed in MFH cases involving extracranial soft tissues.1
One noteworthy aspect of the cases reported in this study is the absence of dural attachment. Both preoperative imaging and surgical findings conclusively established that the tumors were entirely intraparenchymal, devoid of any connection to the dura mater or the cerebral falx. Notably, most intracranial BFH cases reported in the literature have described extra-axial tumors originating from the scalp, cranial bone, or dura mater. In contrast, only four cases have been reported as intra-axial (Table 1). Intriguingly, these intra-axial BFHs, including our cases, shared a favorable prognosis following tumor resection, with no evidence of recurrence. This intriguing observation could suggest that intra-axial BFHs may have distinct biological characteristics compared to their extra-axial counterparts originating from the dura mater. Nevertheless, given the limited number of reported cases for both intracranial MFH and BFH, a comprehensive understanding of their cellular and molecular characteristics is imperative to elucidate whether dural and intraparenchymal BFHs are consistent in their molecular profiles.1
- Clinical features of intracerebral BFH. BFH- benign fibrous histiocytoma (BFH), M- male, F- female, R- right, L left
Conclusion
In summary, we present two exceptionally rare cases of intracerebral BFH without dural adhesion. The MRI findings exhibited non-specific characteristics, with the diagnosis relying on histopathological and IHC examinations. Total surgical excision has been affirmed as the most effective treatment. Rigorous long-term follow-up is crucial to identify any possible tumor recurrence or metastatic progression.
Acknowledgement
We would like to thank Madjaden Inc for English language editing.
Footnotes
Disclosure. The authors declare no conflicting interests, support or funding from any drug company.
- Received December 3, 2023.
- Accepted June 4, 2024.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.