Abstract
Objective: To evaluate the efficacy and safety of carbamazepine, pregabalin, and venlafaxine in patients with painful diabetic neuropathy (PDN).
Methods: Our study was performed as a randomized, double-blind, parallel-group clinical trial between December 2012 and December 2013 at Kermanshah University of Medical Sciences, Kermanshah, Iran. Two hundred and fifty-seven patients with clinically definite PDN were randomized to receive, carbamazepine, venlafaxine, or pregabalin. The primary outcome was subjective pain as assessed by the visual analogue scale (VAS). Secondary outcomes consisted of sleep, mood, and work interference assessments, and a percentage of patients achieving at least 50% reduction in pain intensity.
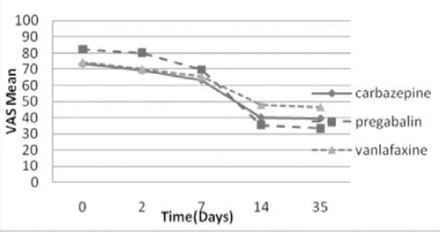
Results: Means of VAS scores for carbamazepine, pregabalin, and venlafaxine treatment groups at the baseline (74.5, 82.3, and 74.5) and endpoint (39.6, 33.4, and 46.6) revealed significant reduction, although pregabalin was more efficacious than carbamazepine, and venlafaxine. Improvements in means scores of sleep, mood, and work interferences were identified in all treatment groups.
Conclusion: This study showed the efficacy of venlafaxine, pregabalin, and carbamazepine in pain reduction in patients with diabetic neuropathy, although pregabalin was shown to be superior to carbamazepine, and venlafaxine in relieving pain, no significant superiority was shown between carbamazepine, and venlafaxine.
Painful diabetic neuropathy (PDN) is a significant microvascular complication of diabetes, affecting 20-24% of diabetic patients.1-4 The pain is often chronic and can be debilitating.5-7 In addition, the increasing prevalence of type 2 diabetes is anticipated to increase the burden of shooting pain in the lower limbs, and feet.8,9 It has major implications on quality of life (QOL), morbidity, and costs from a public health perspective.10 Neuropathic pain is difficult to treat, and patients rarely experience complete pain relief.11 The first step in the management of PDN is glycemic control and correction of any other metabolic disturbances. In addition to controlling hyperglycemia, patients often require management of their pain symptoms.12 The major classes of drugs used to treat PDN are antidepressants and antiepileptics.13 Because of the increasing evidence for effective treatments of neuropathic pain, it is important for the clinician to know which drugs are most effective in relieving pain and associated with the fewest adverse effects, and there is a need for head-to-head studies to guide the clinician in making therapeutic decisions. The aim of this study was to compare the relative efficacy of these 3 therapies in the management of pain in patients with painful diabetic polyneuropathy to provide an appropriate treatment option in such patients.
Methods
The study was performed as a randomized, double-blind, parallel-group clinical trial between December 2012 and December 2013. Our cases were selected from diabetic patients with a diagnosis of diabetic polyneuropathy referred to the diabetic clinic of Taleghani Hospital, affiliated to Kermanshah University of Medical Sciences, Kermanshah, Iran. The diagnosis of diabetic polyneuropathy was confirmed according to the Boulton et al criteria.14 The local ethics committee approved the protocol of the study, and the study was carried out according to the Principles of the Helsinki Declaration. Written informed consent was obtained from all patients. The patients were included based on the following inclusion and exclusion criteria.
Inclusion criteria
1. Diagnosis of metabolically stable type 1 or 2 diabetes with PDN according to the Boulton et al criteria.14 2. History of neuropathic pain for at least 3 months. 3. Patients who had a visual analogue scale (VAS) score15 of 40 mm or more. 4. Age of 18 years old or more.
Exclusion criteria
1. Suffering from ischemic pain and other types of pain unrelated to diabetic neuropathy such as phantom pain due to amputation or arthritis. 2. Electro convulsive therapy in the past 30 days. 3. Use of sedative treatments, hypnotics, anticonvulsants, capsaicin in the past 7 days, and non-steroidal anti-inflammatory drugs or dextromethorphan in the past one day. 4. Use of antipsychotic drugs, monoamine oxidase inhibitors, specific serotonin reuptake inhibitors, opioids, muscle relaxants, and other antidepressants 14 days prior to involvement in the trial. 5. Dependency to alcohol or other drugs. 6. History of diabetic ketoacidosis, nonketotic hyperosmolar condition, or seizure. 7. Pregnancy, lactation, or inability to use contraceptives throughout the study for females of childbearing age. 8. Patients with serious medical conditions such as cardiovascular diseases, thyroid disease, significant hematological diseases, decreased renal (clearance creatinine ≤ 60ml/min) or hepatic function, severe depression (Beck score >13 with Beck Depression Inventory), bipolar disorder, psychosis, history of suicide attempt, or hypersensitivity to study drugs.
Study design
By a computer generated randomization schedule, eligible patients were randomized as follows: the first group received 100 mg carbamazepine (Sobhan Daru, Rasht, Iran) every 12 hours during the first week, then 200 mg every 12 hours until the end of the study, and the second group received 75 mg per day pregabalin (Sobhan Daru, Rasht, Iran) during the first week, then 75 mg every 12 hours, which also continued until the end of the study. Venlafaxine (Sobhan Daru, Rasht, Iran) was administered 75 mg/day during the first week, then increased to 150 mg/day and continued until the end of the study in the third group. Investigators and patients were blind to the treatments by preprinted medication code labels. The patients received the doses for a 4-week period and returned for follow-up at days 2, 7, 14, and 35. During the study period, patients were allowed to take acetaminophen with a maximum dose of 4 g/day. The primary outcome was subjective pain as assessed by the Visual analogue Pain Intensity (VAS-PI), and rated daily by patients. Patients’ daily ratings were tabulated by a researcher for calculation of mean scores at the beginning of the trial and at 2, 7, 14, and 35 days during the trial. The pain VAS is a continuous scale comprised of a horizontal or vertical line, usually 10 centimeters (100 mm) in length, anchored by 2 verbal descriptors, one for each symptom extreme.
Secondary outcomes consisted of quality of life assessments and a percentage of patients achieving at least 50% reduction in pain intensity. The interference of pain with sleep and mood and patients normal work (including both work outside the home and housework) was assessed by Brief Pain Inventory (short form)16 in the first and last visit. The Brief Pain Inventory is an 11-point Likert scale (0: no interference; 10: completely interferes). At the beginning of the trial, general and neurological examination was performed in all patients and also during each visit, vital signs, weight, and ECG were assessed, and a complete physical examination was performed and drug adverse events were evaluated. Glycated hemoglobin and lipid profile was measured at first visit, and complete blood count and differentiation, liver function test, urea, creatine, and sodium were carried out before treatment and after 35 days to detect any hematological or hepatic or biochemical complications. Fasting blood sugar (FBS) and blood sugar 2 hpp (2 hour postprandial) were measured on the first and last visit. If the result of pretreatment hepatic tests was twice the normal range, the patient was excluded from the study, and when an increase in liver function test occurred at the end of the study the medication was discontinued.
Statistical analysis
Patient’s demographic and clinical information were recorded in a predesigned checklist. We estimated a total sample size of 255 patients accounting for 10% drop out assuming a reduction of 50% of VAS between the groups considering 5% level of significance and 80% power. The data were analyzed with the Statistical Package for Social Sciences software version 19 (SPSS Inc., Chicago, IL, USA). Demographic variables and efficacy parameters of 3 groups were compared. Descriptive statistics were used to report variables of each medication group and also for total participants. Post hoc one-way analysis of variance (ANOVA) was used for comparison between the groups. Comparison for pain score on VAS across time in all the 3 groups was carried out using repeated measures ANOVA. Paired sample t-test was used for comparing mean scores of sleep, mood, and work interference across time in each group. Results were reported as mean ± standard deviation (SD), and statistical significance was recognized at p-values <0.05.
Results
Patient distribution
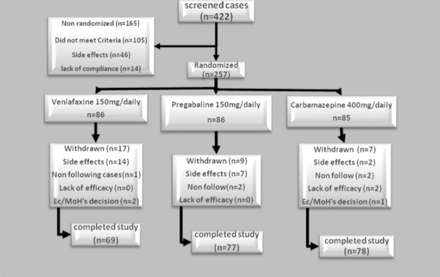
Two hundred and fifty-seven of 422 patients with diabetic neuropathy admitted to the diabetic clinic were randomized. There was no significant difference between baseline clinical and demographic characteristics of the different drug groups (p>0.05) except for VAS (Table 1). The baseline VAS in the pregabalin group was significantly different compared with carbamazepine and venlafaxine (p=0.0001). However, there was no significant difference between carbamazepine and venlafaxine. Of the 257 patients who received medications, 33 subjects dropped out of the study. These included 17 from the venlafaxine, 9 from the pregabalin, and 7 from the carbamazepine groups. In all patients, the main causes for discontinuation of the study were occurrence of adverse events. A flow chart of patient enrollment and distribution is shown in Figure 1.
Baseline demographic and clinical characteristics of diabetic peripheral neuropathy patients.
Distribution of diabetic peripheral neuropathy patients. Ec/MoH - European Commission/Ministry of Health
Efficacy
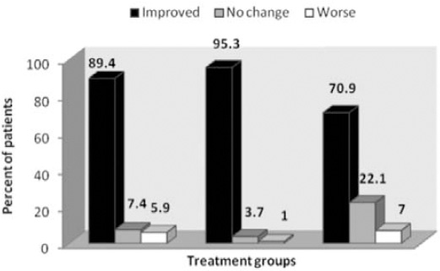
The reduction of pain severity in the 3 groups was statistically significant (p=0.0001) (Table 2). The time course of VAS-PI ratings is demonstrated in Figure 2. Analysis of mean pain scores indicated significant superiority of pregabalin over carbamazepine and venlafaxine as early as day 14 (Table 2). The significant difference in mean pain scores between pregabalin and the other 2 drugs was sustained over days 14-35 (Table 2). There was no statistically significant difference between carbamazepine and venlafaxine in mean pain scores (p=1.00). A statistically significant proportion of patients treated with pregabalin were responders (≥50% reduction in mean pain score from baseline to endpoint) compared with patients receiving carbamazepine and venlafaxine (p=0.004, Table 3, Figure 3).
Means of VAS scores in treatment groups of diabetic peripheral neuropathy patients during the follow-up period.
Comparisons of pain severity scores (VAS) between the treatment groups across time among diabetic peripheral neuropathy patients.
Change in pain severity among diabetic peripheral neuropathy patients.
Change in pain severity in the treatment groups among diabetic peripheral neuropathy patients. Improved and worse categories includes cases with VAS reduction and increase. VAS - visual analogue scale
Sleep, work, and mood
At the end point, mean scores of work, mood, and sleep interference were significantly decreased in all 3 groups (p=0.0001). The reduction of mean sleep and work interference scores in the pregabalin group were significantly higher than the carbamazepine and venlafaxine groups at day 35 (p=0.0001), but no significant difference between the carbamazepine and venlafaxine groups was seen (p=0.270). Pregabalin and venlafaxine were superior to carbamazepine on the mean scores for the pain related mood interference at day 35 (p=0.0001), but there is no statistically significant difference between pregabalin and venlafaxine (p=0.82) (Table 4).
Mean scores of sleep, mood, and work interference in the treatment groups during the follow-up period.
Safety and tolerability
Adverse events were reported by 11 (12.9%) patients in the carbamazepine group, 55 (63.9%) in the venlafaxine group, and 63 (73.2%) in the pregabalin group (p=0.01). Most adverse events were generally mild to moderate in severity. These events seemed to generally resolve over time. The most common side effects were somnolence, nausea, and dizziness. Treatment-emergent adverse events are presented in Table 5. However, the discontinuation of therapy due to adverse event was significantly more common in the venlafaxine group (p=0.01) (Figure 1). Twenty-eight patient that received first dose venlafaxine, 16 patients that received pregabalin, and 2 patients that received carbamazepine withdrew due to adverse events and were substituted with an equal number. Two patients in the venlafaxine group were admitted to hospital due to hypertension and changes in the ECG and were excluded. There were no serious adverse events occurring within any treatment group.
Reported adverse events during and at the end of follow-up among diabetic peripheral neuropathy patients.
There was no significant difference between the 3 groups and among each group in FBS and blood sugar 2hpp in first and last visit (p=0.23).
Discussion
We found that carbamazepine 200 mg/twice a day, pregabalin 75 mg/twice a day, and venlafaxine 75 mg/twice a day could significantly reduce pain intensity, but pregabalin was more efficacious than carbamazepine and venlafaxine. A meta-analysis was performed in 2008, in several studies pregabalin was used to treat 1510 patients.17 The results showed that the drug is effective in a dose-dependent pattern. Prominent pain relief compared with placebo at doses of 150, 300, and 600 mg were achieved. At a dose of 600 mg on the fourth day, and a dose of 150 mg per day on the thirteenth day, pain improvement was reported,17 and The Number Need To treatment (NNT) for a 50% reduction in pain was reported as 4 at a dose of 600 mg/day.18 Two studies evaluated the efficacy of venlafaxine.19,20 One study reported a moderate effect of venlafaxine, with 23% more pain relief than with placebo on VAS-PI scale and an NNT of 5.19 In another study,20 a combination of venlafaxine and gabapentin revealed a moderate effect in relieving pain on 11-point PDN, with 18% more relief than with placebo plus gabapentin. Another study21 showed that venlafaxine was better tolerated and had fewer drug interactions than tricyclic antidepressants. In a study on 49 patients with diabetic neuropathy,22 carbamazepine was administered and an improvement in symptoms was seen, but no changes were observed on the nerve conduction study. A study comparing the efficacy and safety of carbamazepine 100 mg twice daily with venlafaxine 25 mg twice daily on PDN7 showed that the efficacy of venlafaxine was better than that of carbamazepine in reducing the duration of pain (p=0.001), while in our study there is no significant difference between carbamazepine and venlafaxine.
Our study revealed that each of these 3 drugs had a desirable effect on working ability, sleep, and mood of PDN patients, although pregabalin was more effective than carbamazepine and venlafaxine in reduction of work and sleep interference scores, and also venlafaxine and pregabalin were superior to carbamazepine in mood improvement. Similarly in 4 studies that evaluated pregabalin,18,23-26 quality of life measures, social functioning, mental health, body pain, and vitality improved, and sleep interference decreased (all changes p<0.05). Devi8 showed that patients treated with pregabalin have a greater reduction of pain and sleep interference scores compared with patients received duloxetine and gabapentin. In Jai et al’s study,7 they showed that venlafaxine was superior to carbamazepine in improving quality of life. In our study, the maximum pain reduction was seen on the fourth visit (fourteenth day) in all groups. In Jai et al’s study,7 maximum pain reduction was also seen in the seventh and fourteenth days. The most common adverse events associated with these drugs were dizziness and somnolence. The highest frequency was seen in the pregabalin group, and the lowest in the carbamazepine treated patients. In Jai et al’s study,7 discomfort, dizziness, and somnolence were the most common side effects, and also the percentage of adverse events in the venlafaxine group was higher than that in the carbamazepine group. The number of patients withdrawn due to adverse events was significantly higher for venlafaxine in comparison with carbamazepine and pregabalin, both when taking the first dose of medication as well as during study period (p=0.01), and the most common adverse events resulting in the discontinuation of the drug were dizziness, somnolence, and nausea. In our study, venlafaxine was administered as 75 mg/day during the first week, and then increased to 150 mg/day. In the study conducted by Jai et al,7 venlafaxine was effective with doses of 25 mg/twice a day. Several clinical practices have suggested that lower doses of certain drugs, such as warfarin, propranolol, tricyclic antidepressants, and lithium, should be prescribed for Iranians to reach optimal therapeutic levels of these drugs, compared with therapeutic doses required for Europeans and Americans.27-31 Starting venlafaxine and pregabalin at a lower dose at bedtime, and gradually increasing the dose to achieve an effective dose can resulting in drug tolerability. Considering the effects of the 3 drugs compared with the placebo group in previous studies, and to avoid depriving patients from treatment for DNP, this study was performed without a placebo group.
Study limitations
Limitations of this study include: being single centric, absence of any objective criteria to follow-up individuals such as nerve conduction studies, and vibration perception threshold measurements, lack of placebo group, use of the single dose of study medications, the low number of patients in each group, and the short follow-up period.
In conclusion, this study showed the efficacy of venlafaxine, pregabalin, and carbamazepine in pain reduction in patients with diabetic neuropathy. Although pregabalin were shown to be superior to carbamazepine and venlafaxine in relieving pain, no significant superiority was shown among carbamazepine and venlafaxine. It should be noted that due to the complicated pathophysiology of diabetic neuropathy, non-pharmacological treatment of neuropathic pain should be considered, and glycemic control is the most important strategy to prevent and control this condition.
- Received February 11, 2014.
- Accepted April 29, 2014.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.