Abstract
Objective: To assess the efficacy of surgical decompression <24 (early) versus 24-72 hours (late) in thoracic/thoracolumbar traumatic spinal cord injury (TSCI).
Methods: A randomized controlled trial (RCT) of 35 T1-L1 TSCI patients including early (n=16) and late (n=19) surgical decompression was conducted in the neurosurgery department of Shahid Rajaee Hospital from September 2010. Pre- and postoperative American Spinal Injury Association (ASIA) Impairment Scale (AIS), ASIA motor/sensory scores, length of hospitalization, complications, postoperative vertebral height restoration/rebuilding and angle reduction, and 12-month loss of height restoration/rebuilding and angle reduction were evaluated.
Results: Sixteen patients (46%) had complete TSCI. No AIS change was seen in 17 (52%) patients. Complete TSCI patients had no motor improvement. The AIS change in this group was solely due to increased sensory scores. For incomplete TSCI, the mean motor score improved from 77 (±22) to 92 (±12) in early, and from 68 (±22) to 82 (±16) in late surgery. One deep vein thrombosis was observed in each group. There were 2 wound infections, one CSF leak, one case of meningitis, and one decubitus ulcer in the late surgery group. Six screw revisions were required.
Conclusion: Our primary results show overall AIS and motor score improvement in both groups. Motor improvement was only observed in incomplete TSCI. Two-grade improvements in AIS were seen in 3 early, and one late surgery patient.
In traumatic spinal cord injury (TSCI), surgical decompression has been implemented to improve neurological outcomes.1 Clinical benefits of early versus late surgery remain controversial due to lack of well-designed well-executed prospective, randomized controlled trials (RCTs). In this context, the role and timing of spinal decompression after acute TSCI remains one of the most controversial topics pertaining to spinal surgery. Regarding level of TSCI, most studies have been on the cervical area. Moreover, there have been few prospective cohorts systematically examining surgical patients undergoing decompression earlier than 24 hours in cervical spinal cord injury.1,2 The only RCT in this regard compared a <72 hours versus >5 day surgical treatment protocol.3 We conducted an RCT evaluating the efficacy of surgical intervention in thoracic/thoracolumbar (T1-L1) TSCI. In this primary report, we evaluated the relative effectiveness of early (<24 hours) versus late (24-72 hours) decompressive surgery in 35 selected T1-L1 TSCI patients. In the current prospective RCT, a 24 hour cutoff was adopted to define early versus late decompressive surgery based on the best available evidence in this regard.4 Clinical changes in motor and sensory examinations and radiographic outcomes of vertebral height and angle restoration were evaluated. We also assessed the impact of surgical timing on in-hospital postoperative complication rates and mortality.
Methods
Study population and design
We conducted a prospective RCT in a single trauma center, the neurosurgery department of Shahid Rajaee Hospital, affiliated to Shiraz University of Medical Sciences, Shiraz, Iran based on a previously published protocol.5 Patient enrollment began in September 2010 and will continue until a sample size of approximately 328 is reached.5 Ethical approval was obtained prior to enrollment. Written informed consent was obtained from the patients’ authorized representative before performing protocol-specific procedures.
Patients meeting the following criteria were included: age of 18 years or older, TSCI between T1-L1, hemodynamic stability, evidence of spinal cord/conus medullaris compression and/or MRI signal change, and hospital admission before 24 hours of injury. Subjects meeting any of the following were excluded: major and current psychiatric illness, significant concurrent traumatic brain injury, major concurrent medical disease, pre-injury major neurologic deficits or disease, ankylosing spondylitis, penetrating thoracolumbar injuries, pregnant females, life-threatening injuries preventing early cord decompression, criminals under indictment, or incarceration, substance abuse, an American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade of E, no cord compression on MRI, spinal shock, any cognitive deficit, inability to provide informed consent, and an injury involving more than 2 adjacent vertebral levels. The diagnosis of TSCI was based upon history and ASIA criteria. On admission after resuscitation and neurologic assessment, patients were assessed for suitability according to predefined inclusion/exclusion criteria. A preoperative MRI and CT scan were obtained for all patients. Selected patients were randomly divided into 2 groups: Early, undergoing decompressive surgery less than 24 hours from injury, and late, undergoing surgery between 24-72 hours. Specifications of surgical intervention, such as direction of approach and number of levels decompressed were decided by a single attending spinal surgeon.
One of 5 surgical procedures was used: 1) Long-segment including: Pedicle screws inserted at 5 levels; 2 levels above, 2 levels below, and in the fractured vertebra as a fixation point. 2) Long-segment non-including: Pedicle screws inserted at 4 levels; 2 levels above, 2 levels below, but not inside the fractured vertebra. 3) Short-segment including: Pedicle screws inserted at 3 levels; one level above, one level below, and in the fractured vertebra. 4) Short-segment non-including: Pedicle screws inserted at 2 levels; one level above and one level below, but not inside the fractured vertebra. 5) 360º procedure: A 2-stage operation involving a posterior decompression followed by an anterior approach 1-2 weeks afterwards. Posterior decompression was followed by insertion of Titanium vertebral body replacer or tricortical iliac graft. Bone fusion was performed by a mixture of patients’ bone fragments and tricalcium phosphate. The screw placement was evaluated 8-24 hours after each operation by spiral CT scan and revised immediately if the location or alignment were not acceptable.
Standard spinal immobilization and resuscitation techniques were performed. Patients received intravenous methylprednisolone (30mg/kg bolus over 15 minutes, and 5.4mg/kg/h infusion over 23 hours 45 minutes if they arrived <3 hours, and for 47 hours if they arrived 3-8 hours post-injury or even if they arrived later) based upon recommendations from the National Acute Spinal Cord Injury Studies (NASCIS),6 confirmed by a recent systematic review.7 Gastrointestinal prophylaxis was prescribed. Neurologic examinations were performed on admission, preoperatively, immediately after surgery, and at one, 3, 6, and 12-month follow-ups. Primary outcome measures were changes in AIS, and summed ASIA motor and sensory scores. The AIS is a 5 point ordinal scale, classified from A to E, to categorize motor and sensory impairment in individuals with SCI.8 All examinations were performed by blind unbiased examiners not involved in the patient management. The patients and their surgeons were not blinded. Thus, the study was single-blinded. Complications were identified and documented as secondary outcome measures during the patients’ hospital stay and on subsequent follow-ups.
Randomization and masking
Blocked-sample-randomization was used to generate a randomization list. The permuted block method of randomization for a block size of 4 was used. Complete and incomplete TSCI patients each had separate blocks of sample randomization and were assigned to one of 2 groups, early or late. For each patient, an e-mail identifying the treatment was sent to the supervising attending by the principal investigator (PI) of the RCT. Upon patient referral, the supervising attending opened his special e-mail for the first time, printed the treatment protocol, and wrote the patient’s name. The PI supervised commitment to the randomization process by reviewing the printed e-mails and patients’ files, names, and detailed data, which were scanned and sent back confirming that the patient would receive the treatment.
Height/angle restoration
Vertebral height restoration/rebuilding was defined as mean increase in vertebral height a week after surgical decompression expressed in percent calculated as:
[(Height of fractured vertebra postoperatively-height of vertebra preoperatively)/(Predicted vertebral height-height of vertebra preoperatively)]*100
Predicted vertebral height=(height of vertebral body above fractured + height of vertebral body below fractured)/2
Loss of restoration height was defined as mean decrease in vertebral height at 12-month follow-up calculated as:
[(Height of vertebral body one week postoperatively-one-year height)/(predicted vertebral height-height of vertebra preoperatively)]*100
Angle reduction was defined as mean reduction in kyphotic angle during the first week postoperatively expressed in degrees. Loss of angle reduction was defined as mean increase in kyphotic angle at 12-month follow-up compared with postoperative measurement. The same calculations were performed for reduction of kyphotic angle and loss of correction/restoration/rebuilding of angle of fractured vertebrae using Cobb angles.9
Statistical analysis
Analyses were performed using the Statistical Package for Social Sciences version 16.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared using student t-test, or Mann-Whitney test, as appropriate. Categorical data were analyzed by Fisher’s exact or chi-squared tests. A p-value <0.05 was considered as the level of significance.
Results
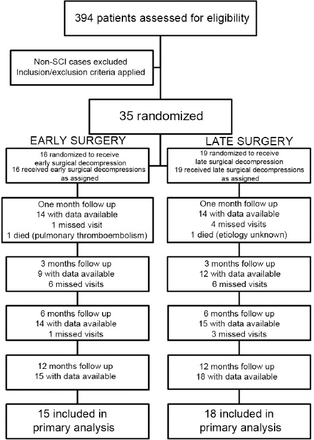
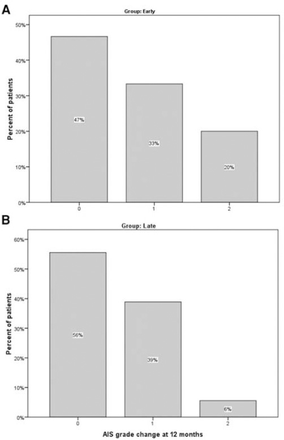
Of 1480 patients referred to the trauma center, 394 had traumatic spinal fracture and were screened for enrollment. Thirty-five patients met our inclusion/exclusion criteria and were included in this study. Sixteen were randomly assigned to early, and 19 to late surgery. Details of patient selection, randomization, and follow-up are presented in Figure 1. There was one death in each group. Table 1 demonstrates baseline patient characteristics, and the Magerl classification of injuries based on pathomorphological criteria10 and surgical techniques employed, all of which were balanced between study groups. In both groups, most injuries consisted of motor vehicle accidents and falls. The most common injury levels were T12 and L1. All patients who underwent a 360 degree operation were adequately decompressed after the initial posterior approach and showed no signs of residual anterior compression upon surgery. Detailed characteristics of each patient are shown in Table 2. Overall, 16 patients (46%) initially had complete TSCI (AIS A). The number of patients with baseline AIS B was 6, with C was 5, and with D was 8. Twelve-month follow-up of patients revealed AIS A in 13, B in 2, C in 5, D in 6, and E in 7 patients. A breakdown of AIS grade improvement is presented in Figure 2. Fifty-three percent of early surgery patients, and 44% of late surgery patients had an improvement in AIS grade. Twelve patients improved one grade, among whom 5 underwent early, and 7 underwent late surgery. Three early surgery patients improved 2 grades, one from B to D, and the 2 others from C to E. One late surgery patient improved 2 grades from B to D. In the early surgery group, one out of 6 patients with AIS A had one grade improvement. In the late surgery group, one out of 9 patients with AIS A had one grade improvement. No change in AIS grade was seen in 17 patients (52%), out of which 13 remained AIS A, one remained C, and 3 remained AIS D. Nevertheless, out of 13 patients who remained in grade A, 7 experienced an increase of 2 or more levels in non-sacral sensory function. Mean length of hospital stay was 7±7.13 days for early, and 9.7±8.28 for late surgery (p>0.05) (Table 1).
Flow diagram of traumatic spinal cord injury patient selection, randomization, and follow-up.
Patient baseline and group characteristics among traumatic spinal cord injury patients.
Characteristics of traumatic spinal cord injury patients enrolled in the study.
American Spinal Injury Association Impairment Scale (AIS) Grade Improvement at 12 months: A) early versus B) late surgery in traumatic spinal cord injury patients.
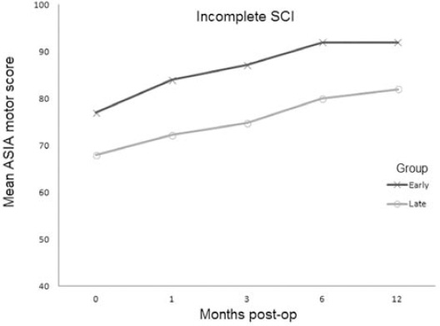
For cases with complete TSCI, no improvement of mean ASIA motor score was observed in either group over 12 months. For incomplete TSCI, both groups showed improvement on follow-up visits (Figure 3). In the early surgery group, the mean motor score improved from 77(±22) on preoperative examination, to 92(±12) on 12-month follow-up visit. In the late surgery group, a change from 68(±22) to 82(±16) was observed. Two cases of deep vein thrombosis were observed, one in each group. In the late surgery group, there were 2 cases of wound infection, one case of CSF leak, one case of meningitis, and one case with decubitus ulcer. A total of 250 screws were used for surgical fixation, 6 of which required revision. In the early surgery groups there was one right-sided T6 revision and a bilateral revision at T9. In the late surgery group, there was one right-sided T12 revision and also one left T9 and right T7 revision both performed in the same patient. All revisions were performed on the same day and were due to lateral placement of screws without cord compression. There was one case of bilateral rod fracture, and screw pulled-out in the late surgery group one year after surgery. Table 3 demonstrates mean restoration/rebuilding of vertebral height and reduction of kyphotic angle one week after surgery and loss of restoration/rebuilding of height and reduction of kyphotic angle after one year. There were no significant differences in radiologic outcomes between groups.
Mean American Spinal Injury Association (ASIA) motor score at baseline and over 1-month, 3-month, 6-month, and 12-month postoperative (post-op) follow-up visits in incomplete traumatic spinal cord injury (SCI) and early versus late surgery groups. No change in motor score was observed in patients with complete traumatic spinal cord injury.
Description of restoration/rebuilding of vertebral height and reduction of kyphotic angle one week after surgery, and one year loss of restoration/rebuilding between groups among traumatic spinal cord injury (SCI) patients.
Discussion
Despite the few prospective studies on surgical decompression in acute SCI, clinical benefits of early or late surgery remain controversial. A prospective analysis indicated no clinical and neurological benefits of surgical decompression between the first 72 hours after cervical SCI and later than 5 days post-injury.3 The relatively late spinal cord decompression, and the 21% loss to follow-up of patients were 2 concerns for the above-mentioned study. However, another level-2 evidence study suggested that surgical decompression earlier than 8 hours would provide better neurological outcome, shorter hospital stay, and lower frequency of secondary complications in comparison with undergoing surgical intervention 3-15 days after thoracolumbar SCI.11 Recently, a prospective cohort compared the effectiveness of early (<24 hours) versus late (≥24 hours) decompressive surgery after cervical TSCI, the results of which favor early surgery.1 A smaller prospective Canadian study2 supported these results, reinforcing the association between early surgery and improved neurologic outcomes.
Our main purpose in conducting this RCT was to evaluate the efficacy of early versus late surgical intervention in the setting of thoracic/thoracolumbar TSCI, as carried out in retrospective studies.12,13 We considered a 24-hour cutoff recommended for early surgical SCI decompression.14 We assigned patients with acute T1-L1 TSCI with documented spinal cord compression to the early/late decompression groups.
Most of the reported literature on TSCI comes from western countries; however, these injuries are potentially more disabling in developing countries.15,16 Feasibility studies have shown that due to transport and life saving measures, only 23.5-51.4% of TSCI patients can undergo an operation within the first 24 hours of injury.17,18 These figures are even more limited in developing countries due to lack of fundamental resources.13 The benefit of surgery timing is unclear, and only a minority of patients may actually receive a 24 hour surgery time frame with current measures in developing countries. Considering the above along with the benefit to society from the scientific answer to timing of surgery, the positive impact that this knowledge may have in allowing for appropriate resource utilization and future planning outweighs the risk of delayed surgical intervention of greater than 24 hours in our study. Furthermore, paraplegia is more common than tetraplegia in TSCI patients in developing countries,19 possibly due to delayed transportation and limited access to full capability facilities for managing cervical TSCI. Therefore, most TSCI patients referred to our trauma centers are cases with thoracolumbar injury. The spinal cord is more sensitive to trauma than lumbar nerve roots,20 and cord injury is more prevalent than root injury in spine trauma.13
We found an increase in AIS grade in both groups. Despite similar baseline characteristics, surgical profiles, and vertebral restoration profiles, a 2-grade improvement was observed in 3 patients undergoing early versus one undergoing late surgery. Although this limited observation is not statistically justifiable, it is in line with recent findings favoring early surgical intervention in SCI. The lower number of complications observed among the early surgery group also follows this trend. The Surgical Timing in Acute Spinal Cord Injury Study1 showed that decompression <24 hours was associated with improved neurologic outcomes defined as at least a 2-grade AIS improvement over 6-month follow-up. However, the authors tempered the conclusions given the intrinsic limitations of the cohort study design.
High-dose steroid therapy is the only pharmacologic therapy shown to have efficacy on neurologic recovery in SCI. Considering the effect of methylprednisolone administration, all our patients were treated according to the recommendations of the NASCIS-2 study.21 Therefore, although methylprednisolone administration may be a major confounding factor for differences in recovery, we believe its effects to be balanced among our study groups.
The ASIA motor score is considered a more reliable predictor of functional outcome after SCI than sensory score.22 No change in motor score was observed in any of our patients with complete TSCI. The AIS change in these patients was solely attributable to an improved sensory level. Regarding the level of TSCI, there may be a predominant component of lower motor neuron, rather than upper motor neuron injury among most of our patients. The chance of neural recovery would be potentially greater than the proximal cervical and upper thoracic spinal cord.23 Improved AIS grade may therefore be ascribed to root escape. Furthermore, regarding the efficacy of treatment, the mid-thoracic region is the most difficult to evaluate. In cases of small caudal improvement in the injured thoracic cord, motor evaluation is difficult. The large distance from the mid-thoracic to cervical and lumbar enlargements makes the efficacy of small positive changes to the motor system almost unrecognizable.24 A retrospective evaluation of T2-T11 SCI showed that surgical decompression has no apparent neurologic benefit in complete TSCI.12 Also, a retrospective evaluation of blunt T12-L1 TSCI revealed no correlation between decompression timing and motor improvement.13
With further regard to ASIA motor score, determination of a functionally meaningful threshold to document the benefit of therapeutic intervention depends on the level and severity of TSCI, as well as degree of spontaneous recovery with conventional treatment.25 In our study protocol, a 5-point or greater improvement in summed ASIA motor score was considered significant.5 On 12-month follow-up of incomplete TSCI, we observed a mean improvement of 15(±10) scores in early, and a 14(±6) score improvement in late surgery patients. Also of note is the fact that the absolute difference in the number of ASIA motor points between groups is not as important as whether a statistically valid difference is present and if the magnitude of the difference confers with clinical benefit and improved functional outcome.26
One of the strengths of the current study is that all surgical procedures were performed under supervision and decision of a single attending. Nevertheless, a uniform standard approach could not be considered due to the distinct quality of each case. Therefore, a more pragmatic approach requiring adequate decompression was chosen. Also, separate randomization of complete and incomplete T1-L1 TSCI enables a comparison of outcome measures in these groups with long-term follow-up and a low dropout rate. Neurological examination of our patients is prone to inter-observer variability as patient assessment and follow-up were not performed by a single examiner. A further limitation is the small number of cases preventing us from employing powerful statistical analyses. We limited our results to mainly presenting descriptive data acquired from the groups thus far. The role of surgical intervention in T1-L1 TSCI can more clearly be determined through advancement and completion of our RCT.
In conclusion, the primary results of our RCT shows an overall improvement in AIS score and motor score in both groups of early and late surgery. Two-grade improvements in AIS were seen in 3 out of 16 in early, and one out of 19 in late surgery patients. Regarding complete TSCI, no improvement in motor score was found in either group; AIS change in this group was solely due to increased sensory scores. An increase in the number of enrolled patients allowing for powered statistical analysis will shed more light on the role of surgery timing in the management of acute T1-L1 SCI.
Acknowledgments
We would like to thank Professor Bizhan Aarabi for scientific editing of the manuscript. The authors thank Bita Pourmand, Sina Hospital Research Development Center, for editing the manuscript, and Iemen J. Talchegah, Aidin Omidvar, Fahim Baghban, Mohammad Jamali, Navideh Mohebali, Hamed Yazdanpanah, Seyede M. Fallahi, Mehdi S. Sotoudeh, and Mohammad R. Sharifirad, Shiraz University of Medical Sciences, for their assistance with patient follow-up.
Footnotes
Disclosure
This study was supported by grants from Sina Trauma and Surgery Research Center of Tehran University of Medical Sciences, and Shiraz University of Medical Sciences (SUMS).
- Received January 29, 2014.
- Accepted April 15, 2014.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.