Abstract
Objective: To assess the tolerability of propofol (PF) in Wada test in an Arab population with temporal lobe epilepsy (TLE).
Methods: This observational study with consecutive sampling took place in King Fahad Medical City, Riyadh, Saudi Arabia. Nine consecutive patients with mean (+SD) age of 26 (+5.8) years, 6 males and 3 females, underwent Wada test between January 2009 and December 2012. Six of them had left TLE, and 3 had right TLE. Each patient received 10 mg of PF in the internal carotid artery (ICA). Right hemispheric injection was followed by left hemisphere injection after 30 minutes. During the procedure, EEG monitoring showed changes within 5-18 seconds of injection as hemispheric delta slowing. Neuropsychological tests were carried out for localization of memory and language.
Results: We were able to lateralize speech dominance in 8 patients and memory dominance in 6 patients. Peri-procedural complications included transient euphoria (n=1), transient spasm of ICA (n=1), eye pain (n=1), facial pain (n=1), and generalized tremulousness (n=2). None of the patients exhibited a symptomatic drop in blood pressure.
Conclusions: We found that PF is well tolerable for the Wada test, with minimally significant complications, although blood pressure should be closely monitored.
Wada test has been the gold standard for lateralization of speech and memory since 1960, when it was first introduced by Juhn Wada.1 The basic concept of the Wada procedure is to inject an anesthetic agent into the brain (through the carotid artery) to sedate one hemisphere transiently in order to localize the dominant hemisphere.1 Patients with temporal lobe epilepsy (TLE) who are candidates for temporal lobe resection are at risk of postsurgical language and/or memory impairment.2 The best agent used for the Wada test is sodium amytal (SA) due to its low toxicity, high efficacy, and adequate duration of action.3 However, SA is facing worldwide shortage, which necessitated the search for other alternatives.4 Propofol (PF) was proposed as a potential alternative.4 The objective of our study was to assess the tolerability of PF in the Wada test in an Arab population with TLE.
Methods
Patient information
This study was approved by King Fahad Medical City (KFMC) Institutional Review Board (IRB) in Riyadh, Kingdom of Saudi Arabia. This observational study with consecutive sampling took place in KFMC. Nine consecutive patients underwent Wada test between January 2009 and December 2012. All patients had a baseline brain MRI, video-EEG recording, and a pre-surgical neuropsychological assessment composed of Addenbrooke’s Cognitive Examination-Revised (ACE-R),5 handedness questionnaire,6 Stanford-Binet Intelligence scale,7 Hospital Anxiety and Depression Scale (HADS),8 and daily memory questionnaire (currently being tested in another study). These questionnaires were in Arabic, given the language barrier, and were tested before being part of the standard neuropsychological evaluation of all patients admitted to the Epilepsy Monitoring Unit (EMU) in KFMC, especially if they were candidates for epilepsy surgery. Outcome was measured by comparing pre- and post-epilepsy surgery neuropsychological parameters. Looking at the outcomes with no drop in functional eloquence proved its validity. Potentially included patients are those who were diagnosed with TLE and were candidates for temporal lobectomy/lobotomy. Age range of the selected population for the procedure was between 18 and 65 years. Patients were excluded if they had history of coagulopathy, carotid dissection, allergy to the contrast or anesthetic agent, elevated serum creatinine, were not fit for surgery (for example, cardiopulmonary risk, major medical problems), or were unable to communicate efficiently during the procedure (for example, deafness). Patients were also excluded from the procedure if their ACE-R score were less than 67 or if they were mentally retarded as per Stanford-Binet Intelligence Scale. Patient selection was according to the Helsinki Declaration.9 All patients consented and received a detailed explanation on the nature, risks, and benefits of the procedure.
Wada test
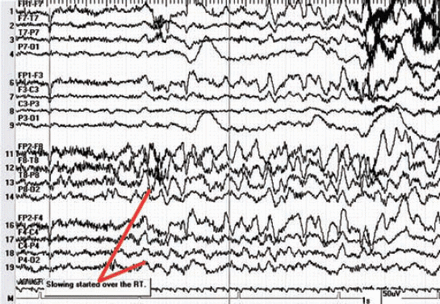
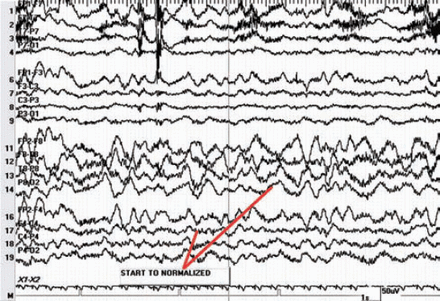
Since SA was not available, PF was chosen as an alternative, based on efficacy, safety profile, feasibility, potential side-effects, and several studies in the literature pertaining to the usage of PF in Wada.10-17 The manufacturer of PF is AstraZeneca, London, England, United Kingdom. The procedure was carried out by a team that included an interventional neurologist, epileptologist, and a neuropsychologist. In each patient, 10 mg bolus of PF was injected into the internal carotid artery (ICA) via a catheter inserted through the right femoral artery, except for one patient who received 15 mg bilaterally. Neither continuous infusion nor maintenance doses were given. We began by injecting the right ICA first, evaluated the left hemisphere, and then injected the left ICA with a 30-minute interval between. The patient was monitored for both motor and electrographic changes. Prior to PF injection, the patient was asked to raise the contralateral arm to observe for weakness clinically, and count from one to 10 in numerical order to observe for speech arrest. In addition, patients were under continuous EEG monitoring to observe for any electrographic changes. The EEG demonstrated high amplitude theta and delta slowing of the ipsilateral hemisphere 5-18 seconds after the injection of propofol (Figure 1). We assessed both hemispheres in the same session with 30-minutes interval between, during which baseline motor, speech, and electrographic activity were restored (Figure 2).
Electroencephalogram paper speed 30 seconds per epoch, high amplitude theta and delta slowing starts on right hemispheric channels, seen 5-18 seconds after injection of propofol.
Electroencephalogram (EEG) paper speed 30 seconds per epoch, EEG starts to normalize, see reduction in amplitude of the slowing.
Lateralization of the language and memory.
The duration of neuropsychological assessment was approximately 2 minutes and 30 seconds. The expressive and receptive language elements were mainly evaluated through counting and performing orders. The patient was shown 5 different objects and was asked to name them. Thereafter, the patient was given 4 different instructions that can be executed. The main elements of memory assessed were both recall (verbal and non-verbal) and recognition. The patient was shown 5 familiar objects and was asked if he/she recognizes them or not. During the test, the patient was shown 5 objects and 3 words and was asked to memorize them. The patient was asked to recall these objects and words as soon as the effect of PF wore-off. These elements were the major determinants of lateralization based on previous studies.10-17
Results
Once PF was injected, we noticed weakness of the contralateral side and aphasia if the involved hemisphere were the dominant one in which the patient stopped counting, could not obey commands, communicate with the neuropsychology team, recognize or memorize objects and words. Motor weakness and aphasia were observed within 5-18 seconds of PF injection. These were simultaneous with EEG changes. Both motor and electrographic changes reverted back to baseline within 10-12 minutes. Out of 9 patients, with mean (+SD) age 26 (+5.8) years, 6 were males, and 3 were females. Eight were right-handed, and only one was left-handed. Six of the right-handed patients had left TLE, and 2 had right TLE. The left-handed patient had right TLE. All patients had MRI findings: 3 patients were found to have left mesial temporal sclerosis (MTS), 2 had right MTS, and 4 had altered hippocampal morphology. Only one patient was documented to have abnormal cognitive functions (Addenbrooke’s Cognitive Examination - Revised 77/100) (Table 1). We were able to lateralize speech in 8 patients, and memory in 6 patients. One patient was left-handed and had bilateral representation of language and memory. All right-handed patients had a left-sided representation of language. However, 2 out of 6 right-handed patients had bilateral representation of memory, while the rest had left-sided representation of memory. All 6 patients with left TLE had language predominance in the same side. From the 3 patients who had right TLE, 2 had left-sided representation of language while one had bilateral representation (Table 1).
Demographic details and summary of results of 9 patients undergoing Wada test.
Adverse effects
One subject experienced behavioral changes after the resolution of PF effect in both sides. He was talkative, curious, disoriented, and speaking incoherently. In addition, 2 subjects experienced shivering during the procedure, and one subject complained of eye pain and facial pain ipsilateral to the site of the ICA injection. The latter developed transient segmental spasm of the ICA. This same patient required an additional 10 mg PF due to lack of effect with the first dose. The ICA spasm occurred only with the first injected dose. The average blood pressure measured for all patients immediately after the procedure was 116/66 mm Hg. None of the patients exhibited a symptomatic drop in blood pressure. The lowest recorded systolic blood pressure was 106 mm Hg. All subjects were able to complete the procedure, testing both hemispheres in the same session. Aside from the mentioned adverse events, no major events were noted.
Discussion
Propofol, an alkylphenol, is a lipid soluble anesthetic agent with rapid onset of action (30 seconds), and rapid rate of distribution (2-4 minutes).18,19 It is metabolized in the liver and excreted mainly through urine.18,19 Propofol was first tried, by Bazin et al20 in 1998, and by Silva et al21 in 2000. Both tried it on a single patient and recorded successful results. In terms of side-effects, the former reported perception of intense blue light, while the latter reported a hot sensation in the head. Thereafter, several studies assessed the efficacy and adverse events of PF. With respect to adverse events, we encountered: shivering, facial pain, and eye pain. Mikuni et al12 performed a case-series of 58 patients in which they studied the side-effects of PF in Wada test. They classified the side-effects into 3 categories: Grade 1 symptoms included pain, shivering, face contortion, lacrimation, laughing, and apathy. Grade 2 symptoms included confusion, involuntary movement, head and eye version. Grade 3 symptoms included seizure-like activity described as increased muscle tone with twitching and rhythmic movements or tonic posturing. Grade 1 and 2 symptoms were also seen with SA.12 Some of these adverse events were also reported by Takayama et al,13 Mikati et al,11 and Magee et al.22 It has been suggested that post-anesthetic shivering associated with PF, although not fully understood, is due to a thermoregulatory phenomenon associated with hypothermia.23,24 As for facial pain and eye pain, it has been proposed that they occur due to peripheral anastomosis and cross-filling between the internal carotid and external carotid arteries.12 A study10 showed that selective middle cerebral artery (MCA) injection of PF was associated with a lower incidence of such side-effects; thus, supporting the “anastomosis” hypothesis. Although we did not encounter any of the grade 3 symptoms in our study, it has been reported that the frequency of grade 3 symptoms increased in patients older than 55 years, after a second injection of PF greater than 10 mg, and/or a total dose of PF greater than 20 mg.12
One of our subjects failed to elicit a response and developed transient segmental ICA spasm. Thereafter, we bypassed the segment of spasm and gave an additional 10 mg of PF, which was successful without any side-effects. Propofol possesses vasodilatory properties,18,19 and this may explain the relatively low systolic blood pressure. Thus, it is probable that vasospasm was secondary to the guide-wire or the catheter (namely, iatrogenic). Mechanically induced vasospasm has been reported in the literature as a complication of angiography, and is mostly self-limited.25,26 Interestingly, a patient developed transient euphoria characterized by talkativeness, curiosity, and was asking many questions. However, there was an element of confusion to this picture and he was speaking incoherently rather than combativeness as reported in previous studies.11-13,22 It has been reported that IV PF might be associated with euphoria, and it might have an addictive effect.27,28 A possible explanation might be an increase in dopamine concentration in the nucleus accumbens, as seen in drug abusers, promoting drug seeking behavior.18,19
In terms of efficacy, it was not possible to compare PF to SA since it was not available. However, we were able to lateralize speech dominance in 8 patients, and memory dominance in 6 patients (Table 1). We also found that the recovery time (both clinically and electrophysiologically) ranged between 10-12 minutes. Three studies11,13,22 compared PF to SA. Takayama et al13 were able to lateralize language and memory in 12 and 9 out of 12 patients, as opposed to 52 and 41 out of 55 patients with SA. They also noticed that the recovery time of muscle power, after the injection, was shorter with PF, while time to verbal and non-verbal response was shorter with amobarbital. Thus, they concluded that PF is a useful alternative to amobarbital in Wada test. Mikati et al11 compared 25 patients injected with PF to 15 injected with amobarbital. They noticed that the time to verbal and non-verbal responses and time to motor power 3/5 were not significantly different between the 2 groups. Magee et al22 retrospectively reviewed the records of 129 patients who underwent Wada test over an interval of 5 years. Interestingly, they did not find a significant difference between PF and amobarbital in regards to length of time to end of hemiplegia, correlated with memory scores, pass/fail rates, and frequency and type of side-effects. In our study, one patient had bilateral representation of language and memory. It has been reported that left-handedness and left-hemispheric lesions increase the likelihood of bilateral representation of language.29 This patient was indeed left-handed and had bilateral MTS.
Other agents have also been studied as potential alternatives for SA in Wada test. These include methohexital, pentobarbital, etomidate, and secobarbital. Secobarbital has been shown to be effective and relatively safe in Wada test.14 Reported side-effects include visual changes and epileptic episodes.14 Moreover, there have been some concerns regarding its availability.3 Methohexital, a rapid-acting barbiturate, has proven to be a successful alternative to amobarbital in Wada test.15 However, methohexital may have potential epileptogenic properties.15 A retrospective chart review study of 760 patients who underwent Wada test by either amobarbital or methohexital concluded that patients with a previous history of epilepsy may be at higher risks of developing seizures after methohexital injection.5 Pentabarbital, a short acting barbiturate, has shown to be equivalent to amobarbital for language and memory lateralization in Wada test. Unfortunately, a patient experienced transient respiratory depression after hyperventilating for 3 minutes.16 Etomidate - a rapid-acting, non-barbiturate, hypnotic agent - showed similar efficacy to amobarbital. Its rapid duration of action necessitates an infusion pump, and maintenance doses.3 Moreover, it has been reported to cause interictal epileptiform activity.17 In addition, it puts patients at risk of adrenal suppression especially if critically ill.30,31
Propofol is a reasonable alternative for SA. Its relatively short duration of action allows us to assess both hemispheres in one session without the need of maintenance doses. Adverse events are mostly benign and reversible. Selective MCA seems to decrease the incidence of adverse events, but requires a highly skilled neurointerventionalist. Precautions should be taken since propofol may induce seizure-like activity, although not encountered in our study. Transient ICA spasm is probably secondary to guide-wire or catheter rather than being caused by PF. But if persistent, Wada should be aborted to avoid cerebrovascular compromise. Blood pressure should also be monitored. Euphoria was an unusual side-effect. Precautions should be exercised in patients with a documented history of bipolar disorder or are predisposed to experience such symptoms. Language lateralization was successfully achieved in most patients. Interestingly, left-handedness and left-sided lesions increase the chance of bilateral representation of language, which carries a prognostic value, in terms of outcome of TLE surgery. The limitations of our study are small sample size, the fact that it is an observational study, and the lack of a control group. A randomized controlled trial with larger sample size is needed to better assess the efficacy and adverse effects of propofol in comparison to SA.
In summary, we believe that PF is a reasonable alternative for SA in Wada test. The relatively short duration of action allowed us to examine both hemispheres in the same session. Side-effects noticed include eye pain, shivering, and facial pain. Selective MCA may decrease the incidence of these side-effects, but requires a skillful neurointerventionalist. In addition, we noticed 2 additional side-effects that were not mentioned in the previous studies;10-13,20-22 euphoria and transient ICA spasm. Transient ICA spasm may be reversible, but if persistent, and severe, Wada should be aborted.
Footnotes
Disclosure
This study was supported by the National Neurosciences Institute, King Fahad Medical City, Riyadh, Kingdom of Saudi Arabia.
- Received October 9, 2013.
- Accepted June 17, 2014.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.