Abstract
Objective: To evaluate the potential therapeutic value of telmisartan (TMT) against diabetic neuropathy (DN) and associated pain in Wistar rats.
Methods: Peripheral DN was induced by a single intraperitoneal streptozotocin injection (55 mg/kg), and 3 weeks later TMT treatment was started (5 and 10 mg/kg/day), and continued for 4 weeks. Mechanical nociceptive threshold, motor coordination, and thermal nociceptive threshold tests were performed before and after TMT treatment. In serum, glucose, pro-inflammatory cytokines including tumor necrosis factor-α, interleukin-1β, and interleukin-6 were assessed. Nerve growth factor (NGF) levels and histopathological changes were estimated in the sciatic nerve. This study was conducted at the Experimental Animal Care Center, Department of Pharmacology, College of Pharmacy, King Saud University, Riyadh, Kingdom of Saudi Arabia between January 2013 and May 2014.
Results: We observed a significant reduction in mechanical nociceptive threshold, motor coordination, and thermal nociceptive threshold in diabetic animals. The TMT treatment significantly enhanced the reduced mechanical nociceptive threshold. The untreated diabetic animals revealed a significant decrease in sciatic NGF, which was markedly attenuated by TMT. The elevated serum levels of cytokines in diabetic animals were inhibited by the TMT treatments. Histopathological evaluation showed obvious nerve degeneration in the diabetic group that was eliminated in the TMT treated diabetic groups.
Conclusion: Telmisartan has a potential neuro-protective effect on peripheral DN; this is mediated through its anti-inflammatory effects and its dual properties as an angiotensin receptor blocker, and a partial peroxisome proliferator activator receptor-g ligand.
Worldwide, diabetic neuropathy (DN) is a major complication of diabetes mellitus. It affects around 15-25% in type-1, and 30-40% in type-2 diabetic patients, causing disabilities, and a high mortality rate. Neuropathic pain defined as a form of chronic pain resulting from damage or abnormal function of the central or peripheral nervous system.1 Patients with neuropathic pain frequently report sensory abnormalities such as burning sensations, hyperalgesia, allodynia, and dysesthesia.2 Diabetic neuropathy can also alter the patient’s quality of life by interfering with emotional well-being, which represents a challenge for clinicians because of its severity, chronicity, and resistance to some classical analgesics.3 The behavioral responses of diabetic rodents to thermal and mechanical hyper- and hypoalgesia as well as tactile allodynia to external stimuli have led to the identification of several mechanisms of abnormal sensation and pain in diabetes. It is confirmed that DN is characterized by neuronal degeneration and marked alterations in neural growth factors such as nerve growth factor (NGF) and insulin-like growth factor (IGF).4 Despite the availability of therapies to alleviate the symptoms of DN, a limited number of medications are available to control its basic causes. Diabetic associated disability and premature mortality are also caused by vascular complications, and several observational reports suggest the potential benefits of intensive blood pressure lowering of diabetic patients.5 The use of angiotensin converting enzyme inhibitors (ACEI) or an angiotensin receptor blocker (ARB) is recommended by current hypertension guidelines for patients with diabetes to achieve a target blood pressure level of 130/80 mm Hg or lower.5 The correlation between the renin angiotensin system and diabetic complications has been observed. Besides being clinically effective in diabetic nephropathy, ACEI or ARBs can improve nerve conduction deficit during peripheral DN in both animal models and human clinical studies. Furthermore, it has been suggested that ARBs are beneficial for nerve regeneration deficits in peripheral DN.6,7 Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a nuclear receptor that activates cellular metabolism leading to cellular growth and differentiation,8 and improved insulin sensitivity.9 The beneficial effects of PPAR-γ ligands were demonstrated in experimental DN by suppressing the angiotensin type receptor 1 (AT1R) expression.10 The PPAR-γ ligands also have anti-inflammatory and antioxidant properties, which are known to be beneficial for microvascular complications in diabetes.9 Telmisartan (TMT) (Micardis®) is one of the most widely used antihypertensives for diabetic patients. It is an ARB with a nephro-protective11 and neuro-protective effect against retinal inflammation.12 Recently, we reported that TMT increases the levels of neurotrophic factors, endogenous antioxidants, and reduces the signs of apoptosis efficiently in diabetic retina.13 The present study was designed to investigate the potential neuro-protective effects of TMT (Micardis®) in a Wistar rat model of peripheral DN of streptozotocin-induced diabetes.
Methods
The present experimental study was designed as an animal diabetic model for neuropathic pain, and completed in the Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia between January 2013 and May 2014.
Animals
Eight to 9 week old male Wistar albino rats (250-280 g) were obtained from the experimental animal care center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Animals were housed under controlled environmental conditions (25°C and a 12 hour light/dark cycle). Animals had free access to pulverized standard rat pellet diet and tap water. The protocol of this study was approved by Research Ethics Committee of College of Pharmacy, King Saud University, Riyadh, Saudi Arabia and was carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (NIH Publications No. 80-23; 1996).
Chemicals and kits
The TMT (Micardis®) was supplied as free samples from Boehringer-Ingelheim Company (Ingelheimam, Rhein, Germany). Streptozotocin (STZ) was purchased from Sigma-Aldrich (Saint Louis, MO, USA). Rat NGF was purchased from USCN LIFE, Wuhan EIAab Science Co., Ltd, (Wuhan, China). Rat ELISA kits for tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 were purchased from R and D systems (Minneapolis, MN, USA).
Diabetes induction
Diabetes was induced by a single intraperitoneal injection of STZ (55 mg/kg) freshly prepared in 0.1 mol/L citrate buffer (pH 4.5).14 Control rats were injected with the same volume of citrate buffer as vehicle. Forty-eight hours after the STZ injection, the fasting glucose levels were detected in a tail vein blood samples using ACCU-CHEK compact plus glucometer (Roche Diagnostics, Meylan, France). Rats with glucose level of 250 mg/dL or higher were considered diabetic.
Study design
Twelve normal rats were divided in 2 groups: 1) Controls (Cont), and 2) TMT (10 mg/kg/day) named (Tel10), and the diabetic rats were randomly divided into 3 groups, 3) STZ, 4) TMT (5 mg/kg/day) named (STZ+Tel5), and 5) TMT (10 mg/kg/day) named (STZ+Tel10). Three weeks after STZ injection, behavioral tests including Randall-Selitto test, Rota rod treadmill. and tail-flick were conducted in all normal and diabetic rats. Then, TMT treatment was started and continued for 4 constitutive weeks. The TMT (Micardis®) tablets were crushed and suspended in 0.25% carboxymethyl cellulose (CMC) solution (Loba Chemie Pvt Ltd, Mumbai, India) and administered to the rats by oral gavage. During the 4 weeks of TMT treatment, the control groups received the same volume of 0.5% CMC. At the end of the treatment, the behavioral tests were conducted again for all groups of animals. After behavioral test, rats were fasted overnight for 12 hours and blood samples were obtained by cardiac puncture under ether anesthesia and then sacrificed by cervical dislocation. Sciatic nerve tissues were extracted and preserved in the deep freezer at -80°C till analysis. Serum samples were separated and preserved at -20°C until analyzed. Serum fasting glucose levels were measured by using the commercially available kit (RANDOX Laboratories Ltd, Crumlin, County Antrim, UK).
Mechanical hyperalgesia (Randall & Selitto method)
For measurement of mechanical nociceptive threshold (Randall-Selitto test) the method described by Sugimoto et al15 was used with slight modifications. A constantly increasing pressure stimulus was applied to the dorsal surface of the rat hind paw while the animal was gently restrained under a soft towel to avoid tissue damage using pressure analgesia meter, Model MK-201D, Muromachi Kikai Co, Ltd., Tokyo, Japan. The pressure was increased until the animal withdrew the paw, squeaked, or struggled. The cutoff pressure was fixed at 400 mm Hg. Three tests separated by at least 10 minute interval were performed for each rat and mean value was used.
Rota rod treadmill test
Treadmill performance was performed by using Rota-Rod Treadmill for rats and mice (Model MK-670, Muromachi Kikai Co, Ltd., Tokyo, Japan) to evaluate the motor coordination of the animals.16 Rats were initially trained to maintain themselves on the rotating rod for more than 2 minutes. In test trials, the time (in seconds) that trained rats could remain on the rod, rotating at 20 rpm, with a cut off at 60 seconds was fixed. Rats were placed on the rotating rod for 2 trials each and scored for their latency to fall in each trial.
Tail flick test
The method described by Sugimoto et al15 was used with slight modifications. Acute nociception was induced using a tail flick apparatus (Tail Flick model DS 20 Sorrel Apelex, Bagneux, France). Briefly, each rat was placed in a restrainer and the tail flick latency was determined by focusing on intensity controlled beam of light on the distal one-third portion of the animal’s tail and recording the time taken to remove the tail from the noxious thermal stimulus. The cutoff time was fixed at 40 seconds to avoid tail injury. For each animal, 2 to 3 recordings were made at an interval of longer than 15 minutes; the mean value was used for statistical analysis.
Estimations of NGF levels in sciatic nerves
Sciatic nerve tissues were homogenized in a cold 50 mM phosphate buffered saline (pH 7.4) by a glass homogenizer (Omni International, Kennesaw, GA, USA). The homogenate was centrifuged at 1000 rpm for 10 min at 4°C to isolate nuclei and unbroken cells. After discarding the pellets, a portion of supernatant was centrifuged again at 12000 rpm for 20 min to obtain post-mitochondrial supernatant. The sciatic nerve level of NGF was assessed and quantified using the enzyme-linked immunoabsorbent assay (ELISA) technique (USCN LIFE, Wuhan EIAab Science Co., Ltd, Wuhan, China) according to the manufacturer’s instructions.
Estimation of pro-inflammatory cytokines in serum
Serum levels of TNF-α, IL-1β, and IL-6 were estimated by commercially available ELISA kits (R and D systems, Minneapolis, MN, USA) following the manufacturer’s instructions.
Histopathology procedure
Sciatic nerve tissue samples were preserved in 10% buffered formalin and processed for routine paraffin block preparation. Using an American optical rotary microtome, sections of thickness around 5 µm were cut and stained with hematoxylin and eosin. The preparations were evaluated by means of bright-field microscope and photographed.
Statistical analysis
The data was expressed as means ± standard error (SE). All statistics were carried out using one-way ANOVA followed by Newman-Keuls post hoc test or using 2-way ANOVA followed by Bonferroni post hoc test. P-values ≤0.05 were considered statistically significant. All statistics tests were conducted using Graph Pad Prism (version 5) software. (GraphPad Software, Inc., La Jolla, CA, USA).
Results
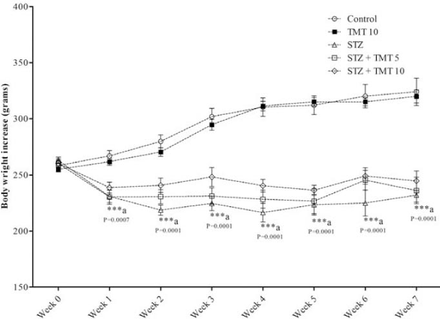
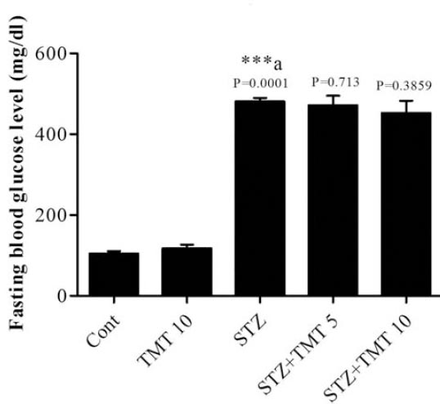
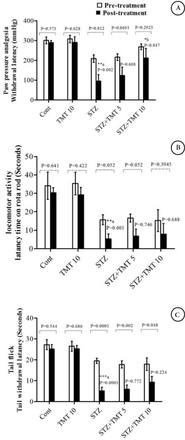
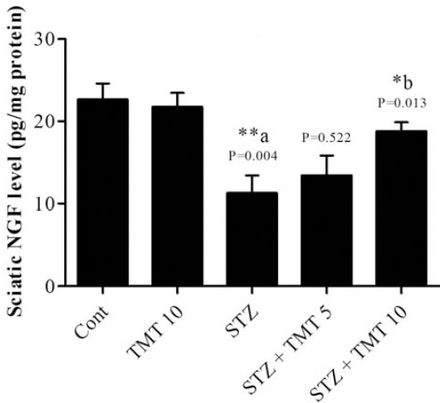
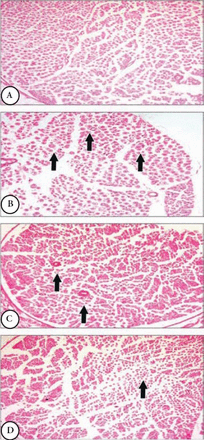
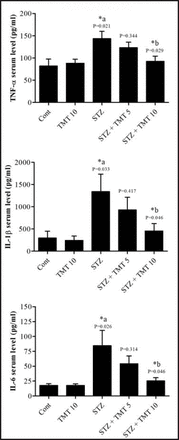
At the beginning of the treatment period, initial mean body weights in all groups were closely equal. Starting from the second week of the experiment until the end of the study, the weights of the diabetic animals significantly (p=0.0001) decreased as compared with control animals. The 4 weeks of TMT administration to the diabetic animals did not attenuate the decrease in animals body weights compared to the untreated diabetic animals (Figure 1). There was a significant (p=0.0001) elevation in the fasting blood glucose levels in diabetic rats compared with control animals. The TMT treatment revealed no inhibition in the elevated blood glucose levels as compared with untreated diabetic animals (Figure 2). Measurement of paw withdrawal pressure 3 weeks after the STZ injection revealed that paw withdrawal pressure did not show a significant difference between non-diabetic and diabetic groups, while it was significantly decreased in the STZ group at the end of the study when compared with the pretreatment STZ values (p=0.012), and post-treatment control group (p=0.002). The TMT (10 g/kg/day) treatment administered to the diabetic rats for 4 weeks significantly (p=0.047) elevated the reduced paw withdrawal pressure values when compared with the untreated diabetic animals (Figure 3A). Results of the Rota rod test showed no significant difference between pretreatment and post-treatment groups. At the end of the study, the STZ group demonstrated a significant (p=0.001) decrease in retention time as compared with the control group. The 4 weeks of TMT treatment administered to the diabetic rats following both the doses did not significantly enhance the Rota rod performance significantly (Figure 3B). Values of tail withdrawal latency 3 weeks after STZ administration (pretreatment), and at the end of the study (post-treatment) in STZ (p=0.0001), STZ+Tel5 (p=0.002), and STZ+Tel10 groups (p=0.018) were significantly different. At the end of the study, tail withdrawal latency was significantly (p=0.0001) reduced in the STZ group when compared with the control group. Both doses of TMT treatment administered to the diabetic animals did not elevate values of tail withdrawal latency (Figure 3C). Sciatic levels of NGF in the diabetic animals were significantly (p=0.004) decreased when compared with control animals. The higher dose of TMT (10 mg/kg/day) markedly (p=0.013) attenuated this reduction when compared with the untreated diabetic rats (Figure 4). Histopathological investigation of sciatic nerve sections from the control group showed benign and normal features of the nerve (Figure 5A). In the STZ-induced diabetic animals, there was a focally scattered loss of myelin sheath, well-formed degenerated bodies, and scattered chronic inflammatory cells infiltrate (Figure 5B). The TMT (5 mg/kg/day) treated diabetic group showed few scattered degenerated nerve bodies associated with inflammation and focal loss of myelin sheath that was considered a mild degree of nerve degeneration (Figure 5C). In the TMT higher dose group (10 mg/kg/day), the sciatic nerve histopathological sections showed a lower degree of inflammatory cells infiltrate and degenerated bodies (Figure 5D). Pro-inflammatory cytokines including TNF-α (p=0.021), IL-1β (p=0.033), and IL-6 levels (p=0.026) in the diabetic rats were significantly increased when compared with the control animals. These elevated levels of pro-inflammatory biomarkers were markedly TNF-α (p=0.029), IL-1β (p=0.046) and IL-6 levels (p=0.046) inhibited after 4 weeks of treatment with TMT (10 mg/kg/day) (Figure 6).
Effect of telmisartan on body weights of normal and diabetic male Wistar albino rats. Data are expressed as mean± S.E and analyzed using 2 way ANOVA followed by Bonferroni post hoc test. Six rats were used in each group. ***acontrol versus STZ group. TMT - Telmisartan, STZ - Streptozotocin, Cont - control, S.E - standard error
Effect of telmisartan treatment on diabetic male Wistar albino rats post-treatment blood glucose levels. Data are expressed as Mean± S.E. and analyzed using one-way ANOVA followed by Newman-Keuls post hoc test. Six rats were used in each group. ***a Cont vs STZ group. TMT - Telmisartan, STZ - Streptozotocin, Cont - control, S.E - standard error
Effect of telmisartan on A) pain threshold in paw pressure analgesia, B) rota rod performance, and C) tail withdrawal latency rate in diabetic and non-diabetic animals. Data are expressed as mean±S.E. (n=6) and analyzed using 2 way ANOVA followed by Bonferroni post hoc test. The significance different compared to post-treated and pre-treated expressed as p-values. ***a, **acomparison between the diabetic and non-diabetic and **bcomparison between TMT treated to diabetic rats to untreated diabetic animals. TMT - Telmisartan, STZ - Streptozotocin, Cont - control, S.E - standard error
Effect of telmisartan on nerve growth factor (NGF) levels in sciatic nerve of normal and diabetic male Wistar albino rats. Data are expressed as mean±S.E. and analyzed using one-way ANOVA followed by Newman-Keuls post hoc test. Six rats were used in each group. **aCont vs STZ group; *bSTZ vs diabetic TMT treated groups. TMT - Telmisartan, STZ - Streptozotocin, Cont - control, S.E - standard error
Effects of telmisartan on the histopathological features of sciatic nerves in diabetic and non-diabetic animals A) normal histology of sciatic nerve fibers in control animal, B) scattered degenerated bodies with loss of myelin sheath and inflammatory infiltrate along the nerve fibers in diabetic untreated group, C) few degenerated nerve bodies with focal loss of myelin sheath in STZ+TMT5 group, and D) lower degree of inflammatory cells infiltrate and degenerated bodies in STZ+TMT10 group. TMT - Telmisartan, STZ - Streptozotocin
Effect of telmisartan on serum levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in diabetic and non-diabetic male Wistar albino rats. Data are expressed as mean±S.E. and analyzed using one way ANOVA followed by Newman-Keuls post hoc test. Six rats were used in each group. *aCont versus STZ group; *bSTZ versus diabetic TMT treated groups. TMT - Telmisartan, STZ - Streptozotocin, Cont - control, S.E - standard error
Discussion
In the present study, we demonstrated that administration of TMT to diabetic rats prevented the progression of DN. Our data revealed that TMT treatment significantly enhanced thermal and mechanical analgesia. Results indicate protection against a diabetic-induced increase in pro-inflammatory biomarkers and inhibited NGF levels. The histopathological evaluation further strengthened the estimated values of biochemical estimations and also justified the protective effect of TMT against diabetic-induced changes.
The development of several diabetic complications, such as retinopathy, nephropathy, and neuropathy have been suggested to be due to hyperglycemia.17 Chemically induced diabetes by STZ in rodents is a well-recognized animal model to investigate several metabolic and pharmacological properties of drugs.18 Prompt pancreatic β-cells damage with marked hyperglycemia was reported after a single intraperitoneal STZ injection administered to animals in the current and other investigations.18 Diabetic animals treated with TMT (5 and 10 mg/kg/day) for 4 weeks did not show a significant decrease in glucose levels compared with untreated diabetic rats. The STZ induced-hyperglycemia significantly provoked a decrease in the mean body weights of TMT treated and non-treated diabetic animals.
Diabetic neuropathic pain is one of the most common diabetic complications that occurs in approximately half of diabetic patients. Experimental induced diabetes by STZ is a well-documented animal model to explore behavioral changes associated with DN.16 Thermal sensitivity assessment in diabetic rats and mice is carried out using a number of behavioral tests such as tail flick and hot-plate tests. Both thermal hyper- and hypo-algesia have been described in STZ-diabetic rats with short-term (2-8 weeks) diabetes.19-22 Behavioral methods to test mechanical hyper- and hypo-algesia in experimental studies include mechanical withdrawal thresholds in diabetic rodents models assessed by paw (rats) and tail (mouse) pressure Randall-Selitto test, or with a von Frey esthesiometer and rigid von Frey filaments.23 Mechanical hyper- and hypo-algesia have been also reported in STZ-diabetic rats.20,21 In addition to spontaneous pain, tactile allodynia, a condition in which light touch is perceived as painful, has been also reported. Tactile allodynia is observed in both human subjects with diabetes mellitus and in STZ-diabetic rats, in which the light touch of von Frey filaments or the light stroking of a paw induces a withdrawal response from the stimulus.24 Furthermore, marked impairment in the muscle coordination of diabetic animals was reported. A significant reduction in the retention time and decreased motor performance of the diabetic animals on the rotating rod were reported in several studies.16,25 In the present study, diabetic animals showed a significant delay in mechanical and thermal latencies as well as retention time on the rota-rode than that observed in control animals, indicating mechanical and thermal hyper-algesia development. These findings agree with other investigations, where the same experimental techniques and animal models were applied.16,26 The TMT treatment administered to the diabetic rats for 4 weeks significantly attenuated the impaired mechanical algesia, but not the altered movement coordination. Moreover, results of the tail flick test revealed that although, the differences between tail withdrawal latencies of diabetic animals were not significant at the end of the study, each diabetic, TMT treated group showed a significant difference from its self-control at the third week of the treatment. Indeed, it is important to notice that there was a descending decrease in the values of significances of the diabetic untreated and treated groups, which might indicate a slight alleviating effect of TMT. The TMT did not significantly prevent the thermal hyper-algesia but it inhibited its development and progression.
Nerve growth factor has a fundamental function for the survival and maintenance of sympathetic and sensory nerves. It has a neuro-protective role and potentiates axonal growth. Alterations in neuronal NGF levels can cause neuronal dysfunction and death.27 In the current investigation, sciatic levels of NGF were markedly reduced in the diabetic animals, indicating loss of neuronal integrity and nerve cells apoptosis. Furthermore, histology of the sciatic nerve of diabetic animals confirmed this neuronal degeneration. The TMT clearly attenuated this degeneration. Sciatic levels of NGF were significantly reduced by the higher dose of TMT treatment (10 mg/kg/day) administered to the diabetic rats for 4 weeks. Also, histopathological investigation of the sciatic nerve section from the STZ+TMT10 group revealed a decrease in degenerated bodies and preservation of sciatic nerve architecture. These findings are in agreement with several other reports that investigated the effect of ARBs on nerve regeneration. An orally active ARB, ZD-7155, prevented nerve regeneration delays in STZ diabetic rats.6 Also, enhanced nerve regeneration was observed after direct application of losartan on crushed sciatic nerves.7 These studies suggest that ARBs might be beneficial for nerve regeneration deficits in diabetic peripheral neuropathy. Telmisartan is a structurally unique ARB as it has a partial agonist of the PPAR-γ.28 Agonists of PPAR-γ are known to affect the regulation of carbohydrate and lipid metabolism29 and expression of several pro-inflammatory genes.30,31 Studies also suggested that PPAR-γ activators can induce neuro-protection.32,33
Hyperglycemia is known to be one of the vital factors that can induce expression of inflammatory biomarkers during diabetes via oxidative stress pathways. A diabetic associated rise in the inflammatory path is known to induce neural cells death and dysfunction.34 The present findings highlight the association between inflammation and the development of DN. The neural degeneration that was demonstrated in the histological analysis of the sciatic section from the STZ group was associated with inflammatory infiltration. Moreover, levels of pro-inflammatory biomarkers such as TNF-α, Il-1β, and IL-6 were increased in diabetic animals compared with controls. It is well known that such inflammatory cytokines play a vital role in systemic inflammation and in propagation of acute phase reaction.35 Indeed, studies suggested that the elevated values inflammatory mediators during diabetes are a consequence of hyperglycemia and insulin resistance.36 The TMT was found to have effective anti-inflammatory properties in several studies.37 Furthermore, Araujo et al38 reported that TMT can suppress the production of several pro-inflammatory biomarkers expressions such as TNF-α, COX-2, MMP-2, and MMP-9. Results of our investigation showed similar findings where the higher dose of TMT (10 mg/kg/d) produced significant inhibition against the elevated pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in experimentally-induced diabetic rats. In addition, diabetic animals treated with the higher dose of TMT showed a lower degree of inflammatory cells infiltrate. These anti-inflammatory effects have been suggested to be through the PPAR-γ activation pathway.37
In conclusion, the present study revealed that TMT alleviated hyperglycemia-associated alterations in mechanical or thermal hyperalgesia, but not motor coordination, and improved DN via its anti-inflammatory and neuro-protective properties in diabetic rats. Both of these properties are suggested to be through PPAR-γ partial activation of the tested ARB. Histomorphological assessments also demonstrated that STZ induced sciatic damage was markedly reduced by TMT administration. Although there is lack of molecular evidence, these findings suggest that TMT might be a beneficial ARB in chronic diabetic patients exhibiting neuropathy. Further investigations are needed to support these findings at the molecular and clinical levels.
Clinical Practice Guidelines
Clinical Practice Guidelines must include a short abstract. There should be an Introduction section addressing the objective in producing the guideline, what the guideline is about and who will benefit from the guideline. It should describe the population, conditions, health care setting and clinical management/diagnostic test. Authors should adequately describe the methods used to collect and analyze evidence, recommendations and validation. If it is adapted, authors should include the source, how, and why it is adapted? The guidelines should include not more than 50 references, 2-4 illustrations/tables, and an algorithm.
Acknowledgments
The authors thank Dr. Hala Elsayed for her efforts and assistance in the histopathological investigation section.
Footnotes
Disclosure
The authors have no affiliation or financial involvement with organizations or entities with a direct financial interest in the subject matter or materials discussed in the manuscript. Funding was received from the Deanship of Scientific Research at King Saud University, research group project No RGP-VPP-263.
- Received August 10, 2014.
- Accepted February 9, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.