Abstract
Objective: To evaluate the therapeutic effects of inhibiting interleukin-1 beta (IL-1β) in vivo using Anakinra in an experimental model of spinal cord injury (SCI).
Methods: All experimental procedures were performed in the animal laboratory of Ankara Education and Research Hospital, Ankara, Turkey between August 2012 and May 2014. The SCI was induced by applying vascular clips to the dura via a 4-level T5-T8 laminectomy. Fifty-four rats were randomized into the following groups: controls (n = 18), SCI + saline (n = 18), and SCI + Anakinra (n = 18). Spinal cord samples were obtained from animals in both SCI groups at one, 6, and 24 hours after surgery (n = 6 for each time point). Spinal cord tissue and serum were extracted, and the levels of IL-1β, malondialdehyde, glutathione peroxidase, superoxide dismutase, and catalase were analyzed. Furthermore, histopathological evaluation of the tissues was performed.
Results: The SCI in rats caused severe injury characterized by edema, neutrophil infiltration, and cytokine production followed by recruitment of other inflammatory cells, lipid peroxidation, and increased oxidative stress. After SCI, tissue and serum IL-1β levels were significantly increased, but were significantly decreased by Anakinra administration. Following trauma, glutathione peroxidase, superoxide dismutase, and catalase levels were decreased; however, Anakinra increased the activity of these antioxidant enzymes. Malondialdehyde levels were increased after trauma, but were unaffected by Anakinra. Histopathological analysis showed that Anakinra effectively protected the spinal cord tissue from injury.
Conclusion: Treatment with Anakinra reduces inflammation and other tissue injury events associated with SCI.
Post-traumatic inflammatory reactions may play an important role in the secondary injury processes that occur after spinal cord injury (SCI).1,2 New treatment strategies like Anakinra aim to block or attenuate the critical mediators of inflammation in ischemia and reperfusion damage after spinal cord injuries. Primary traumatic mechanical injury to the spinal cord may cause neuronal death with irreversible recovery or regeneration. Neurons continue to die for several hours after SCI; however, this neuronal death could potentially be prevented. A large number of biochemical, and molecular cellular interactions result in secondary neuronal death. One of these interactions is the local inflammatory response in the injured spinal cord. It is thought that microglial cells might be the source of cytotoxic cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β), which kill oligodendrocytes. Within one hour after SCI, increased synthesis, and/or secretion of IL-1β, is detectable at the injury site. Interleukin-1β is a member of the IL-1 cytokine family. The gene encoding this cytokine, and 8 other IL-1 family genes, form a cytokine gene cluster on chromosome 2.3 The mentioned cytokine is produced due to the activation of macrophages as a proprotein; the active form is produced secondary to the proteolytic action of caspase 1. The IL-1β is an important mediator of the inflammatory response, and is involved in a variety of cellular activities including cell proliferation, differentiation, and apoptosis.3 Anakinra is shown as an IL-1 receptor antagonist blocking the inflammation and cartilage degradation effects of naturally occurring IL-1 in rheumatoid arthritis, by competitively inhibiting the binding of IL-1 to the IL-1 type receptor.4 The IL-1 is produced in response to inflammatory stimuli and mediates various physiological responses including inflammatory and immunological reactions. In patients with rheumatoid arthritis, the natural IL-1 receptor antagonist is not found in sufficient concentrations in the synovium and synovial fluid to counteract the elevated IL-1 concentrations. Anakinra is considered a “biological response modifier” rather than a “disease-modifying antirheumatic drug” because it is able to selectively target the pathological elements of the disease.5 For this study, we determined the following endpoints of the inflammatory response: 1) histological damage, 2) cytokine expression (IL-1β), and 3) measurement of lipid peroxidation and oxidative stress (glutathione peroxidase [GPx], malondialdehyde [MDA], and superoxide dismutase [SOD]).6 The aim of the present study was to evaluate whether Anakinra administration could protect the spinal cord from lipid peroxidation and oxidative stress after SCI in rats.
Methods
Animals
Fifty-four adult male Wistar albino rats weighing 300-350 g (age 4-5 months) were used in this study. All experimental procedures were approved by the Ethical Committee of Ankara Education and Research Hospital to minimize animal discomfort during surgery and recovery. The study was carried out in the Animal Laboratory of Ankara Training Hospital, Ankara, Turkey between August 2012 and May 2014.
Surgical procedure and sample preparation
All procedures involving laboratory animals were performed according to the National Institutes of Health Guiding Principles in the Care and Use of Animals. The surgical procedure was performed under general anesthesia induced by intraperitoneal (i.p.) xylazine (10 mg/kg; Bayer, Istanbul, Turkey) and ketamine hydrochloride (60 mg/kg; Parke-Davis, Istanbul, Turkey). A longitudinal incision was made on the midline of the back, exposing the paravertebral muscles. These muscles were dissected away, exposing the T5-T8 vertebrae. We used the clip compression model described by Tator and Fehlings.7 The spinal cord was exposed via a 4-level T5-T8 laminectomy, and SCI was produced by extradural compression of the spinal cord for one minute using an aneurysm clip (standard aneurysm clip, FE751; Aesculap, Tuttlingen, Germany) with a closing force of 24 g. After surgery, 1.0 mL of saline was administered subcutaneously to replace the blood volume lost during the surgery. After the surgical and traumatic interventions, the surgical wound was closed in layers with silk sutures. During recovery from anesthesia, the rats were placed on a warm heating pad and covered with a warm towel. The animals were housed in a group at an ambient temperature of 24 ± 1°C with a 12-hour light-dark cycle for a survival period of 24 hours. The animals were allowed free access to water and food ad libitum. After surgery, the bladders of the animals were manually voided. All the animals were anesthetized with the above-mentioned agents at 24 hours post-trauma, and their spinal cords (2 cm) were immediately extracted without any damage.
Experimental groups
Fifty-two rats were randomly allocated into the following groups and subgroups: Control: control rats (n = 18) underwent laminectomy, and non-traumatic spinal cord samples were obtained soon after surgery. Trauma: SCI + saline rats (n = 18) were subjected to SCI and received a single i.p. dose of one mL/kg saline. Traumatic spinal cord samples were obtained one (n = 6), 6 (n = 6), and 24 hours (n = 6) after surgery. Anakinra: SCI + Anakinra rats (n = 18) were subjected to SCI and received a single i.p. dose (100 mg/kg) of Anakinra (Anakinra, Swedish Orphan Biovitrum AB, Stockholm, Sweden) immediately following SCI. Traumatic spinal cord samples were obtained one (n = 6), 6 (n = 6), and 24 hours (n = 6) after surgery.
Cytokine assay
Double-antibody sandwich enzyme-linked immunosorbent assays (R & D systems, Minneapolis, MN, USA) were applied for determining the serum and tissue concentrations of IL-1β according to the manufacturer’s instructions.
Tissue and serum biochemical analysis
Portions of brain tissue were evaluated tissue levels of SOD, catalase (CAT), MDA analysis, and GPx analysis. Furthermore, blood serum levels of SOD, CAT, MDA, and GPx analysis, were measured.
Histopathological procedures
For histological examination, spinal cord tissue samples were fixed in 10% neutral buffered formalin, dehydrated through a graded series of ethanol, and embedded in paraffin. Then, 5-µm–thick paraffin sections stained with hematoxylin-eosin were analyzed and photographed with light microscopy (Olympus CX21, Olympus America Inc., Melville, NY, USA). In all groups, the degenerated neurons in the gray matter and histopathological changes after SCI were scored between 0-3 based on the following: 0 = no damage; one = one to 5 eosinophilic neurons in the gray matter, mild neural tissue damage; 2 = 5 to 10 eosinophilic neurons in the gray matter, moderate neural tissue damage, and white matter vacuolization; and 3 = more than 10 eosinophilic neurons in the gray matter, severe neural tissue damage, and hemorrhage. All animal specimens were evaluated by 2 observers who were blinded to the groups.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences software version 19.0 for Windows (SPSS Inc., Chicago, IL, USA). Non-parametric tests were applied, and the Mann-Whitney U test was used to compare 2 independent groups while the Kruskal-Wallis test was used to compare more than 2 groups. The Wilcoxon Signed Ranks Test was used to compare 2 dependent groups while the Friedman Test was used to compare more than 2 groups. Bonferroni correction for multiple tests was used for post-hoc comparisons. All differences associated with a chance probability of 0.05 or less were considered statistically significant. Continuous variables were presented as mean±SD.
Results
Tissue and serum IL-1β analyses. Table 1 summarizes the changes in tissue and serum IL-1β levels. There were statistically significant differences between the trauma, control, and Anakinra groups at one hour after trauma with regard to mean tissue IL-1β activity (p=0.003). After 6 hours, we observed decreased tissue IL-1β activity in the Anakinra-treated group, but this difference was not statistically significant (p=0.459). We compared the trauma, control, and Anakinra groups at 24 hours after trauma with regard to mean tissue IL-1β activity. There was no statistically significant difference observed (p=0.366), although the tissue level of IL-1β in the Anakinra-treated group continued to decline. There were statistically significant differences between the trauma, control, and Anakinra groups at one hour after trauma with regard to mean serum IL-1β activity (p=0.003). After 6 hours, we observed decreased serum IL-1β activity in the Anakinra-treated group, and this difference was statistically significant (p=0.03). At 24 hours after trauma, the mean serum IL-1β activity of the trauma, control, and Anakinra groups were significantly different (p=0.002) (Table 1).
Changes in tissue and serum interleukin-1 beta (IL-1β) levels in an SCI experimental animal model.
Tissue and serum MDA analyses
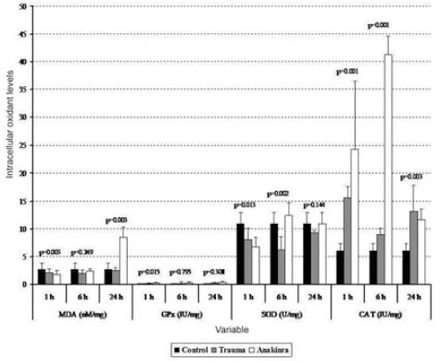
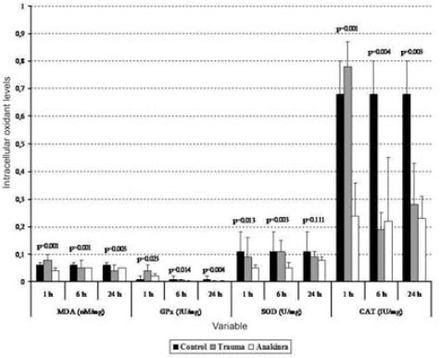
The mean serum MDA levels in the trauma and Anakinra groups were compared with those in the control group, and there was a statistically significant difference observed between the groups at all time points (p<0.05). When comparing the mean serum MDA levels in the trauma and Anakinra groups to the levels in the control group, there was no statistically significant difference observed between the Anakinra and control groups at any of the time points (p>0.05) (Figures 1 and 2).
Tissue biochemical alterations in the 3 groups. Values are expressed as mean ± SD or median (interquartile range), where applicable. Times indicate the timepoint after injury in hours. CAT - catalase, GPx - glutathione peroxidase, MDA - malondialdehyde, SOD - superoxide dismutase
Serum biochemical alterations in the 3 groups. Values are expressed as mean ± SD or median (interquartile range), where applicable. Times indicate the timepoint after injury in hours. CAT - catalase, GPx - glutathione peroxidase, MDA - malondialdehyde, SOD - superoxide dismutase
Tissue and serum GPx analyses
The mean tissue GPx levels at one hour after trauma were compared between the trauma, control, and Anakinra groups, and a statistically significant difference was observed (p<0.001). After 24 hours, the mean tissue GPx levels continued to decline, but this decrease was not statistically significant (p=0.308). When the mean serum GPx levels in the trauma and Anakinra groups were compared with those in the control group, there was a statistically significant difference observed between the control groups at all time points (p<0.05) (Figures 1 and 2).
Tissue and serum SOD analyses
The mean tissue SOD levels in the trauma and Anakinra groups were compared with those in the control group, and a statistically significant difference was observed between the groups at all time points (p<0.05). The mean tissue SOD levels in the trauma and Anakinra groups were compared with the control group, a statistically significant difference was observed between the groups at all time points (p<0.05) (Figures 1 and 2).
Tissue and serum CAT analyses
The mean tissue CAT levels in the trauma and Anakinra groups were compared with those of the control group, and a statistically significant difference was observed between the groups at all time points (p<0.05). When the mean serum CAT levels in the Anakinra group were compared with those of the control group, a statistically significant difference was observed between the groups at all time points (p<0.05) (Figures 1 and 2).
Histopathological assessment
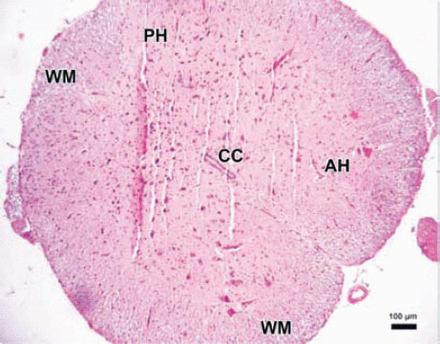
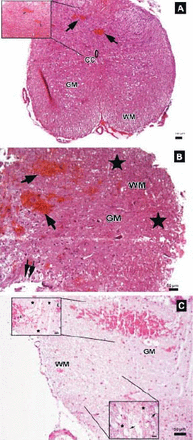
The parenchymal features of the white and gray matter, neuronal morphology, and vascular structures appeared normal in the control group (Figure 3). Areas with tissue damage and hemorrhage in both the gray and white matter were noted in spinal cord trauma and Anakinra applied after trauma at one, 6, and 24 hours. Mild to moderate edema and neuronal injury with hemorrhagic areas in both the gray and white matter was shown. In the trauma groups; spinal cord sections showed prominent disturbances between the boundaries of white and gray matter (Figure 4). Swollen astrocytic extensions formed clear spaces around the injured neurons (Figure 5). The neuropil showed spongiosis due to the vacuolization of neuronal and glial cellular extensions. In the gray matter, injured neurons displayed central chromatolysis, which was distinguished by cytoplasmic hyalinization and peripheral displacement of Nissl bodies (Figure 6). White matter fiber bundles showed edema, myelin degeneration, and axonal dilatation in all trauma and Anakinra-treated groups at all time points. Although there were no differences between the histopathological scores of the trauma and Anakinra-treated groups at one or 6 hours, the histopathological score of the Anakinra-treated group was improved compared to that of the trauma group at 24 hours (Figures 4 and 7).
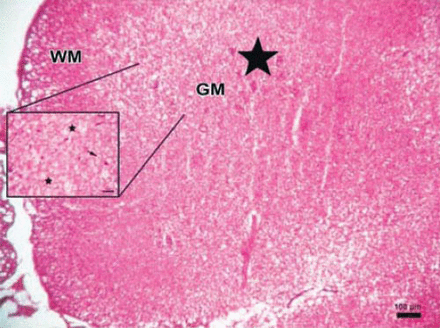
Photomicrograph of the control group. The parenchymal features of the white (WM) and surrounding gray matter, neuronal morphology, and vascular structures appeared normal in the control group (Hematoxylin & Eosin staining ×4). PH - posterior horn, CC - central canal, AH - anterior horn
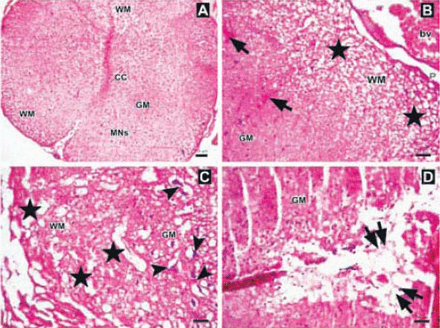
Photomicrographs of the trauma group at all time points. A) There was mild to moderate edema, and prominent disturbances were seen between boundaries of white (WM) and gray matter (GM), central canal (CC), and hemorrhagic areas in GM (arrows) (Hematoxylin & Eosin [H&E] staining, ×4). Inlet showing higher magnification of hemorrhagic areas (arrows) (H&E ×40) B) Hemorrhagic areas in GM (arrows), cavitation (double arrows), and dilatation and edema in WM fiber bundles (stars) (H&E ×10). C) Serious neuronal loss and hemorrhagic areas (H&E ×10). Upper left inlet showing hemorrhage (E) and spongiosis (stars) in GM (H&E ×40). Lower right inlet showing edema (stars), axonal degeneration and dilatation of WM fiber bundles (arrows) (H&E ×40).
Photomicrographs of the Anakinra-treated group one hour after trauma showing A) regions and structure of spinal cord gray matter (GM), white matter (WM), central canal (CC), multipolar neurons (MNs) (Hematoxylin & Eosin [H&E] staining ×4). B) Edema, axonal dilatation and degeneration in WM (stars), and hemorrhagic areas in GM (arrows), (H&E ×10). C-D) Swollen astrocytic processes forming clear areas around degenerated neurons (arrowheads), axonal dilatation and degeneration (stars), and central cavitation around CC (double arrows)(H&E ×10). bv - blood vessel, P - pia mater
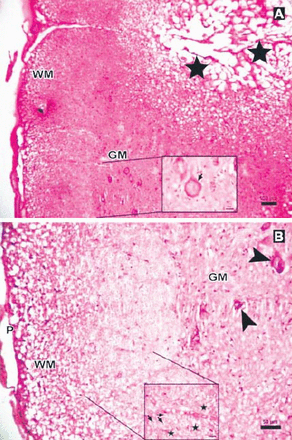
Photomicrographs of the Anakinra-treated group at 6 hours after trauma showing less edema and fewer degenerated neurons showing: A) central cavitation (arrowhead, Hematoxylin & Eosin staining [H & E] ×10) B) Eosinophilic neuron (thick arrow, H & E ×40). P - pia mater, GM - gray matter, WM - white matter
Photomicrograph of the Anakinra-treated group at 24 hours after trauma showing less edema and neurons (stars) with intensely stained basophilic cytoplasm in gray matter (GM), (Hematoxylin & Eosin [H&E] staining ×4). Inlet showing axons with myelin sheath (arrow), degenerated axons and dilated fibers (stars) (H&E ×40).
Discussion
Following a spinal ischemia period, oxidative stress may be evaluated by glutathione, SOD, and MDA activities.8,9 Following the initial ischemia period in the first 24 hours after SCI, neuronal damage is supposed to continue for several days.10,11 Shortly after SCI, in several hours, proinflammatory cytokines such as IL-1β and TNF-α aim to regulate the precise cellular events in that area. This expression continues for several days in the neuronal tissue, and can be detected in microglia, perivascular macrophages, and astrocytes.12,13 Harrington et al14 demonstrated that increased neuronal expression of IL-1β and its receptors occurs 6 hours after acute SCI. They also demonstrated that IL-1β levels in the CSF were significantly higher than the levels found in the CSF of sham-injured animals at one hour after SCI, indicating that this cytokine is released into the interstitial fluid. In this study, we demonstrated that serum and tissue IL-1β levels were the highest at 24 hours after trauma without Anakinra treatment. Rothwell12 showed that neuronal inflammation, which induces procytokine IL-1β, plays a key role in this process. Additionally, the elevation of serum and tissue IL-1β levels after SCI involves glial and invading immune cells. Lu et al15 recently demonstrated that IL-1 production is elevated after spinal cord ischemia and is maintained for up to 3 days after reperfusion. Administration of an IL-1 receptor antagonist attenuates the severity of SCI.16 Here, we histologically demonstrated significant neuronal injury in rats exposed to spinal trauma. In contrast, administration of Anakinra remarkably attenuated the neuronal injury associated with the decreased tissue and serum IL-1β levels, and improved the histopathological examinations. Our results show that treatment with Anakinra attenuates the amount of IL-1β in the injured spinal cord, as well as decreases the oxidative stress, infiltration of neutrophils, and spinal cord damage. In contrast, no significant reduction in MDA levels, which indicate inhibition of lipid peroxidation, was observed in the spinal cord sections or serum levels obtained from the Anakinra-treated rats. This observation is in disagreement with the study by Marini et al,17 which clearly demonstrated that inhibiting lipid peroxidation reduces IL-1β expression and protects neuronal tissue from damage. To confirm the therapeutic effect of Anakinra, we evaluated thin spinal cord sections using a light microscope. We observed that the serious neuronal loss, gliosis, central cavitation, necrosis, and diffuse hemorrhagic areas were reduced in SCI-operated rats treated with Anakinra at 24 hours after trauma. These findings suggest that IL-1β plays a detrimental role in the development and severity of post-traumatic injury. However, the harmful effects of IL-1β can be attenuated by blocking its signaling pathways. We demonstrated only a few of the possible mechanisms by which Anakinra attenuates neurological injury.
Here, we report for the first time that treatment with Anakinra inhibits IL-1β after SCI and prevents serious neuronal loss and gliosis. The limitation of the study is the absence of the reports of Anakinra for long term controlled studies and the need for accurately adjusting the dosage scheme. A better understanding of the action mechanisms of Anakinra might help establish potential clinical therapeutic strategies for SCI practice in the future.
As a result, we can conclude that the destructive characteristics of SCI on spinal cord tissue and neurons may favorably be attenuated by IL-1 receptor antagonist Anakinra therapy. In particular, Anakinra may improve the outcomes of SCI by penetrating into the cerebrospinal fluid especially in the first 24 hours of spinal trauma besides its beneficial effects in the treatment of rheumatoid arthritis. Future clinical studies are needed for better understanding the alternative mechanisms of Anakinra for treating SCI.
COPYRIGHT
Whenever a manuscript contains material (tables, figures, etc.) which is protected by copyright (previously published), it is the obligation of the author to obtain written permission from the holder of the copyright (usually the publisher) to reproduce the material in Neurosciences. This also applies if the material is the authors own work. Please submit copies of the material from the source in which it was first published.
Footnotes
Disclosure
The authors have not disclosed any affiliation or financial involvement with organizations or entities with a direct financial interest in the subject matter or materials discussed in the manuscript. No funding was received for this work from any organization.
- Received July 23, 2014.
- Accepted March 9, 2015.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.