Abstract
Objective: To evaluate the effects of temporal lobe epilepsy (TLE) on corpus callosum (CC) morphometry in patients with TLE.
Methods: This retrospective study was conducted at the Faculty of Medicine, Tekirdag Namik Kemal University, Tekirdag, Turkey between November 2010 and December 2013. The epileptic syndrome diagnosis was based on International League Against Epilepsy criteria, and this study was conducted on the MRIs of 25 epilepsy patients and 25 control subjects. We classified the patients according to their duration of epilepsy <10 and ≥10 years. The projection area length (PAL) of the CC was also estimated. Total brain volumes (TBV) were measured on CT images.
Results: The mean values of TBV for patients with TLE and the control group were not statistically different, but the CC PAL values were statistically different. The mean CC PAL values of under and over 25 years of age in patients with TLE were statistically different. The mean values of TBV of under and over 10 years duration of TLE were small statistically, but the CC PAL values were statistically different.
Conclusion: The results indicate a clear influence of TLE on the structure of the CC rather than TBV.
Epilepsy is associated with comprehensive neurological and metabolic disorders.1 Epileptic seizures are caused by changeable electric discharges in some neuron groups due to short-term brain function disorders. Temporal lobe epilepsy (TLE) is the most common form of symptomatic and adult epilepsy; it starts in the early childhood period. Accumulating evidence has shown that TLE is a disorder of abnormal epileptogenic networks, rather than focal sources.2 The relationship between the cortical brain structure with neuropsychological and neurophysiological events cannot be completely explained.3 The corpus callosum (CC) is the most important tract between cortical brain structures. Recent studies have shown us that a slimming of the CC is seen in patients with epilepsy. The CC is the largest commissural pathway that interconnects the 2 hemispheres of the brain.4 In the literature, the CC is recognized as especially related to age, gender, brain functions, and neurologic diseases.5 In addition, the development of the CC might be altered by environmental factors and pathological changes.3-5 Neuroimaging techniques, such as MRI and CT, are increasingly used for examining anatomical structures and neuropsychological disorders, such as Alzheimer’s disease, dementia, and epilepsy.6 Lately, MRI investigations have indicated that patients with epilepsy may experience a degeneration of cortical structures. These investigations use newly defined and more effective methods for determining the degeneration of cortical structures. Several methods may be used to account for the total volumes of tissue, organs, and their components.7 Stereological methods can be used to analyze anatomical structures when it is impossible to remove organs and tissue. A planimetric procedure is one of the most reliable stereological methods. The planimetric approach is the gold standard for clinicians’ assessments of neurological disorders.2,8 The volume of structures like the CC, which are entirely composed of fibers, cannot be directly determined. Because of this difficulty, projection area length (PAL) is used to define the CC’s projection in the brain. The purpose of this study was to evaluate the effects of TLE on CC morphometry in patients with TLE.
Methods
Adult patients who were radiologically and pathologically diagnosed with TLE were included in this retrospective study. Patients with temporal lobe damage, patients with abnormal morphology of the CC were excluded. This study conformed to the Helsinki Declaration, and was approved by the local clinical research committee at the medical faculty of Namik Kemal University, Tekirdag, Turkey. All patients were under regular and detailed observation between November 2010 and December 2013 in the Neurology Clinic in the Practice and Research Hospital at Namik Kemal University. The diagnoses for epileptic syndromes were based on the International League Against Epilepsy criteria. This study was conducted using the MRIs of 25 patients with TLE (11 males and 14 females) and 25 control subjects (11 males and 14 females). We classified the patients according to their TLE duration: <10 years and ≥10 years. Gender and age (<25 years of age and ≥ 25 years of age was included) were matched for demographic purposes, and the duration of TLE was studied for clinical evaluation. Control groups that had a lifetime history, including any kind of neurological illness, head injury, or pathological disorder, were excluded from the study.
Planimetry methods for estimating projection area length
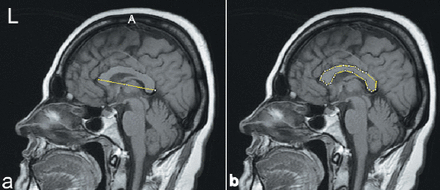
Planimetry is the most commonly used technique for estimating irregularly shaped projection areas. Many researchers estimate organ and tissue volumes using the planimetric method: a cross-sectional area is multiplied by a section’s thickness.8 The MRI was used to evaluate the surface area of the CC using ImageJ software (ImageJ, 1.37v: National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/), which is a public-domain software delivered by the US National Institutes of Health. ImageJ’s estimated results are accepted as the gold standard in scientific studies.8 In this method, both the projection surface area of the structure, and the length of the reference distance of the image was measured. The square of the length was also recorded. The images were transferred to ImageJ software. Of all the sagittal sections, the midsagittal section, where the CC fibers are compact, was selected. The midsagittal brain sections were defined by identifying the interhemispheric fissures in the coronal and sagittal planes (seventh or eighth section). The outline of the CC and all the thicknesses were traced using the digitizer, and the metric scale of the software (Image J version 1.43) was used to take these measurements. First, the straight lines with a known length on the images were used to calibrate the program. Then, the polygon selection tool was used to define the outlying boundaries of the CC (Figure 1).
Measurements of the planimetry technique for the assessment of the projection areas length of the corpus callosum using ImageJ software. a) Indicates is the length of corpus callosum (L), b) the surface area of the corpus callosum, A - indicates the surface area of the corpus callosum, and L - is the length of corpus callosum.
The digitally traced boundaries of the surface areas were automatically calculated for the midline section (7 or 8), and the machine administered the projection area of the CC. The PAL of the CC was also estimated using the following formula, where A indicates the surface area of the CC and L is the length of the CC.8
PAL = A/L2 × 100
Finally, all data were recorded in an Microsoft Excel spreadsheet.
Calculation of cerebrum volume
The planimetry method was used to measure the cross-sectional surface area using Dicom Works software (Version 1.3.5, France; http://dicom.online.fr.) and ImageJ. For the planimetry measurements, pictures were taken from the MRI that held a millimetric scale for the calibration.9 The coefficient error of the planimetric volume estimations was calculated using the following formula, which was described in previous studies:10,11
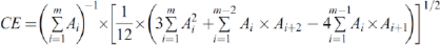
Acer et al11 reported that using a hand-hold mouse, the rater traced around the area of interest within one slice. The system calculated the number of pixels surrounding the traced area, and the process was repeated for each slice. Each measurement was carried out at least 3 times using software tools to the nearest millimeter, and the average was used for the calculation. The mean time for the volume estimations was also provided. Using Microsoft Excel, calculations of cerebrum volume, coefficient error of estimates, and other data were formatted in a spreadsheet.
Statistical analyzes were completed using PASW® Statistics 18 for Windows (SPSS Inc., Chicago, IL, USA). The results are shown as mean ± standard deviation (SD). Distribution of the groups was analyzed with the Shapiro-Wilk test. Independent samples of t-tests were used to compare the mean values of the groups (epilepsy-control). Differences between 2 groups were analyzed using the Mann-Whitney U-test. The limit of statistical significance was set at p<0.05.
Results
This study intended to determine the area covered by the CC in the brain and TBV in both a control group and patients with TLE. Comparisons were made using the CC PAL values and TBV in patients with TLE and the control group. Twenty-five years and older were chosen as myelination of the brain is thought to be complete. The mean values of TBV for patients with TLE and the control group were not statistically different (p>0.05); however, the CC PAL values were statistically different (p<0.0001). Patients with TLE and the control group were divided into 2 groups: individuals under and over 25 years of age. This classification was used to determine the relationship between age, and the size of the CC. In the control group, the mean value of TBV and the CC PAL values of individuals under and over 25 years of age were not statistically different (p>0.05). The mean TBV value for individuals under and over 25 years of age in patients with TLE was not statistically different (p>0.05); however, the CC PAL values were statistically different (p<0.0001) (Table 1).
Demographic data of the control and the patient groups.
Patients with TLE were divided into 2 groups: individuals under and over. This classification was used to determine the relationship between the size of the CC and duration of TLE. The mean values of TBV for individuals duration of TLE of <10 or >10 years were not statistically different (p>0.05), but the CC PAL values were statistically different (p<0.0001) (Table 2). Significant atrophy was observed in the CC of individuals who were 10 years of age or older suffering from TLE. Regression analyzes were performed to show whether there is a relationship between TLE duration and CC atrophy. It was determined that the degree of atrophy depends on the duration of TLE by 33%. Using an ANOVA test, a statistically significant relationship was observed at the value of p<0.01.
Comparison of CC PAL values and TBV in the control and patient groups.
As shown in Table 2, the CC PAL values of both male and female patients with TLE were determined to be smaller than in healthy individuals (p<0.0001, p=0.027); however, this was not the case for TBV (p>0.05). In gender comparisons of patients with TLE, the CC PAL values and TBV of women are smaller than in men (p=0.031, p=0.035).
Discussion
New studies on epilepsy, which investigate etiology, treatments, and various other areas, are frequently being added to the literature. Epilepsy, which is a neurodegenerative disease, directly affects the brain, and cortical structures. It causes a worsening of health conditions, and a decline in quality of life. During epileptic seizures, electrical signals change in the cortical structures. Epileptic seizures may also cause neuronal losses and degeneration in many areas of the brain.2,12,13 Local changes that cause an epileptic focus are now clearly understood because of the developments that have been made in imaging techniques in recent years. Scientists are attempting to find new methods of processing and storing these images. Additionally, changes in cortical structures can be determined using a planimetry method, which is considered the golden standard.2,3,6,12 The CC, which is composed of myelin fibers, is the primary cause for a generalization of seizures generated by an epileptogenic focus. Disconnections between cortical structures may also cause neurological diseases, such as epilepsy or Alzheimer’s disease. Electrical discharges, which cause epilepsy, may affect not only temporal, frontal, or occipital lobes but also other cortical components.14 Although many epilepsy studies concern the temporal and frontal lobe, very few of them address how epilepsy affects the CC. In recent years, many research articles have indicated that CC atrophy could be associated with a loss of function in cortical structures.14-16 There are inconsistent reports concerning the volume of the CC in patients with epilepsy. Firat et al4 and Pulsipher et al16 reported that it was decreased, and O’Kusky et al17 reported that there was no difference in the CC volumes of patients with epilepsy when compared with the CC of healthy subjects. Differences in the CC volumes of individuals with epilepsy may be due to methodological differences in the studies. In our study, the CC PAL value indicates the projected area that is occupied by the CC in the brain and provides us with information on the size of the CC. A few studies have been carried out that measure the CC sizes in epilepsy patients using diffusion tensor imaging and MRI.13-15 No detailed research has been carried out to determine the size of the CC in the brain and decrease in amount of CC. In different studies, CC neuronal cortical atrophy has been observed in epilepsy patients.4,13,15 However, these studies do not clearly express whether this atrophy affects the whole brain. In our study, patients with TLE were determined to have smaller CC PAL values than the control group; this did not hold true for TBV values. These findings suggest that epilepsy leads to a loss of volume in the CC rather than the TBV. Our study examined the effects of gender on TLE patients’ CC size. In the literature, some studies assert that gender does not affect the size of the CC.18,19 However, many other studies have found that gender does affect CC size. For example, Witelson et al20 reported that the CC was larger in males. Pulsipher et al16 also reported that male patients have larger CC volumes than female patients. In our study, females had smaller CC PAL values than males in both the patient group and control group. Therefore, it is unclear whether gender is a factor affecting the CC size in patients with TLE. The differences between the patients and control group were statistically significant for the isthmus and splenium of the CC.4 In our study, we compared the CC sizes and TBV in females and males within each group: the control group, and the TLE group. Both female and male TLE patients had smaller CC PAL values than females and males in the control group; this was not the case for TBV. These results indicate that epilepsy affects the CC size rather than the TBV. In our study, we examined the relationship between age and TLE. Previous studies have shown that the CC size reduces with age. Tang et al21 observed that older females have a smaller CC than younger females. Hermann et al22,23 reported that childhood-onset TLE was associated with a significant volumetric reduction of the CC compared to both late-onset cases and healthy controls. We compared CC size and TBV in individuals under and over the age of 25 for both TLE patients and control groups. There was no difference in CC size and TBV between individuals under and over the age of 25 in the control groups. However, was a difference in CC size between TLE patients under and over the age of 25; this was not the case for TBV. These results indicate that the degree of CC atrophy is higher in patients with TLE who are over the age of 25. This increases after the age of 50. Hutchinson et al24 reported no difference in the callosal volumes of children with new-onset epilepsy, and age-matched controls. Fırat et al4 and O’Dwyer et al15 found that in TLE patients, there is a constant decrease in the CC volume with an increased duration of epilepsy. A significant CC volume reduction was reported by Hermann et al22 for early-onset TLE patients as compared with late-onset patients and controls. In our study, patients who suffered from epilepsy for more than 10 years were determined to have increased thinning in their CC, but not in their TBV. These results indicate that neuronal atrophy accelerates over time and that a slimming of the myelin sheath occurs when epileptic attacks are increased. Given the effects on dysfunction and the development process, increasing neuronal atrophy may cause serious damage in children, such as epileptic encephalopathy and mesial temporal sclerosis.23 The duration of epilepsy may be an important factor in determining the extent of the influence of TLE on the CC. Previous studies reported that decreases in the CC volumes in TLE are related to a lack of non-verbal problem-solving skills and fine motor dexterity.4,22,23 This means that the CC occupies a reduced area in patients with TLE. Additionally, a loss of function may occur in the frontal, temporal, and occipital lobes because of the effects on the CC functions of these lobes.
Study limitations
The modest sample size of patients with TLE was a limitation of our study.
In conclusion, The present study demonstrates a clear influence of TLE on the structure of the CC rather than TBV. The duration of epilepsy and the age of the patient are the most important factors for determining the influence of TLE on the CC. The PAL method is proposed as a simple and practical method for CC evaluations. This method will allow us to carry out more reliable research concerning other cortical structures that cannot be measured using CT or MRI. The goal of future research is to identify any relationship between CC and TLE with a larger number of subjects.
Footnotes
Disclosure
The authors declare no conflicting interests, support or funding from any drug company.
- Received December 15, 2015.
- Accepted February 17, 2016.
- Copyright: © Neurosciences
Neurosciences is an Open Access journal and articles published are distributed under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC). Readers may copy, distribute, and display the work for non-commercial purposes with the proper citation of the original work.